Translate this page into:
Multifocal Balo's Concentric Sclerosis in Children: Report of a Case and Review of Literature
Address for correspondence: Prof. Jayantee Kalita, Department of Neurology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareily Road, Lucknow - 226 014, Uttar Pradesh, India. E-mail: jayanteek@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Balo's concentric sclerosis (BCS) is a rare demyelinating lesion considered to be a variant of multiple sclerosis (MS). On magnetic resonance imaging (MRI) Balo's concentric sclerosis shows the typical concentric pattern. We report a case of 10 year old child with BCS who presented as post infectious acute disseminated encephalomyelitis (ADEM). He is asymptomatic and had no relapse after 6 years of follow-up.
Keywords
Acute disseminated encephalomyelitis
demyelinating
magnetic resonance imaging
multiple sclerosis
INTRODUCTION
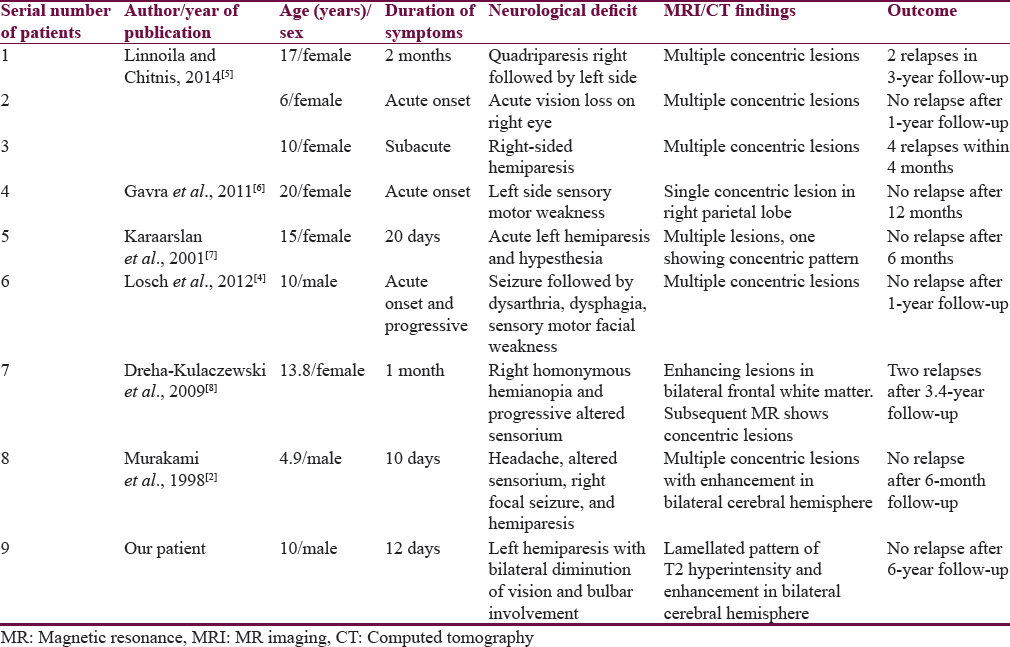
Balo's concentric sclerosis (BCS) is a rare demyelinating disease of the central nervous system (CNS) and considered to be a variant of multiple sclerosis (MS). The CNS lesion consists of characteristic concentric rings of demyelination alternating with myelinated rings in the white matter, sparing the gray matter.[1] Earlier BCS was a fulminant fatal disease diagnosed on autopsy, but now BCS is diagnosed antemortem with the help of magnetic resonance imaging (MRI), and long-term survival with disease regression has been reported.[2] The characteristic cranial MRI features may help in the diagnosis of BCS and avert invasive brain biopsy.[3] In the medical literature, only 8 patients of BCS have been reported in pediatric age group [Table 1].[245678] We report a child with multifocal BCS and review the literature in pediatric patients.
CASE REPORT
A 10-year-old boy presented with weakness of the left side and painless diminution of vision of both eyes (left > right) for 12 days. He had low-grade intermittent fever 20 days prior for 3 days. The patient was treated in the local hospital. Cranial MRI revealed multiple well-defined T2 and fluid-attenuated inversion recovery (FLAIR) hyperintense lesions in the bilateral cerebral hemisphere. Nodular enhancement was noted in postcontrast scan [Figure 1a1–a3]. He received intravenous methylprednisolone 10 mg/kg for 3 days and discharged in a stable condition. On day 10th of illness, he developed acute onset spastic dysarthria that progressed to total mutism in 6 h. He also had dysphagia and drooling of saliva. There was no numbness, paresthesia, alteration in sensorium, or recent worsening of motor power. There was no recent history of immunization. His perinatal history and developmental milestones were normal. There was no history of seizure or any significant family history. His pulse rate was 70/min, blood pressure 110/80 mmHg, afebrile and general physical examination was unremarkable. He was conscious, oriented, and aphasic due to the immobile and spastic tongue. He had left supranuclear facial palsy, and his gag reflex was exaggerated. Vision, pupils, and fundus examination was normal. Motor examination revealed left spastic hemiparesis of Grade 3 on Medical Research Council (MRC) scale with hyperreflexia. Sensations and coordination were normal. Blood counts, hemoglobin, serum chemistry, and cerebrospinal fluid analysis was normal (nil cell, protein 40 mg/dL, and sugar 55 mg/dL). His vasculitic profiles (rheumatoid factor, antinuclear antibody, anti-double stranded DNA, extractable nuclear antigen screen) were either negative or normal. HIV serology and venereal disease research laboratory were negative. Cranial MRI done on day 20th of illness [Figure 1b1–b5]. T2-weighted and FLAIR sequence revealed multifocal alternating rings of hyperintensity and hypointensity in supratentorial white matter bilaterally sparing the gray matter. Perilesional edema was seen around all the lesions without significant mass effect. The concentric lesion showed enhancement on the postcontrast T1-weighted image (T1WI) suggesting synchronous nature of active demyelination. Diffusion-weighted imaging shows circular rings of hyperintensity and restricted diffusion. Magnetic resonance spectroscopy revealed reduced n-acetylaspartate and elevated lactate and choline. Apart from these concentric lesions, no other lesions were noted. As compared to previous MRI, the lesion size was increased and had concentric rings enhancement with surrounding white matter edema. No new lesion was noted. From the characteristic MRI findings, a diagnosis of BCS was made. The patient was managed conservatively with a repeat course of injection methylprednisolone for 5 days and discharged after 10 days with 1-grade improvement in motor power on MRC scale. He improved completely in the next 2 months, and his follow-up MRI after 6 months revealed significant decrease in the size of bilateral frontoparietal lesions that was only evident in T2 and FLAIR images, and there was no postcontrast enhancement. The posterior parietal lesions resolved completely. No evidence of any black hole on T1WI. There was no new T2-hyperintense lesions [Figure 1c1–c3].
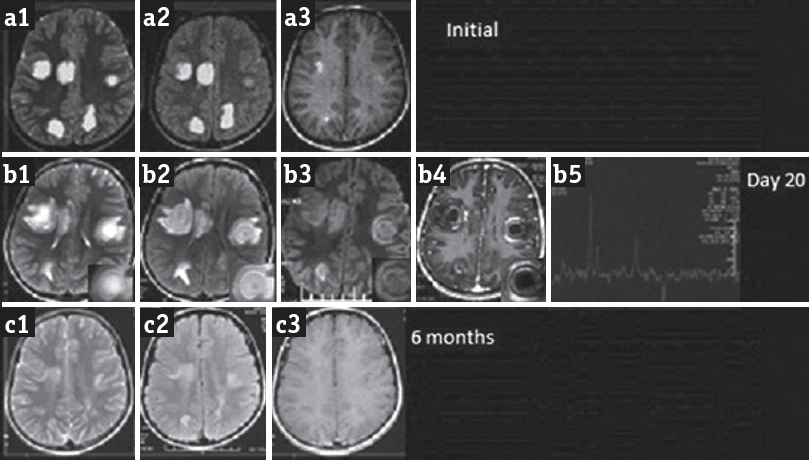
- Cranial magnetic resonance imaging shows multiple well-defined T2 (a1) and fluid-attenuated inversion recovery (a2) hyperintense lesions in bilateral cerebral hemisphere showing nodular enhancement in the postcontrast T1-weighted image (a3). Repeat magnetic resonance imaging on day 20: Axial T2-weighted (b1) and fluid-attenuated inversion recovery (b2, b3) shows multifocal alternate hyperintense and hypointense concentric rings with mild perilesional edema in bilateral cerebral hemisphere showing concentric enhancement on the postcontrast T1-weighted image (b4). Magnetic resonance spectroscopy (b5) shows increased lactate and choline and reduced n-acetylaspartate. Magnetic resonance imaging after 6 months shows significant reduction in size of the lesions (c1, c2) without any contrast enhancement (c3)
DISCUSSION
This child had BCS because of clinical and characteristic MRI findings who responded well to methylprednisolone. BCS is a rare demyelinating disease of CNS classically considered to have a rapid progression and poor outcome. However, recently many cases of benign and nonfatal BCS have been reported.[57] This may be due to the fact that many mild cases of BCS were not recognized before the advent of MRI. Whether this is a variant of MS or a discrete disorder is still a matter of debate. Some consider it to be a rare and acute variant of MS, whereas others consider it to be a separate entity. Brain biopsy is the gold standard for diagnosis of BCS; however, with the advent of MRI, early diagnosis is possible noninvasively by the characteristic lamellated pattern of the lesion.[7] It is more common in young male. Oligodendrocytes are the main target in BCS resulting in necrosis and infiltration of T-lymphocyte, CD20B cells, and macrophages. Recently, the involvement of astroglia and microglia has also been reported with expression of aquaporin-4 and breakdown of gap junction simulating neuromyelitis optica. The association of BCS and ischemic vasculopathy has also been postulated due to report of Notch 3 in BCS.[9] The surrounding white matter with intact myelin has been attributed to preconditioning effect of surrounding oligodendroglia with expression of heat shock protein 70, hypoxia inducible factor, and other protective protein.[10]
BCS has been classified into three subtypes - (1) single self-limited event, (2) relapsing remitting, and (3) primary progressive.[11] Our case had monophasic illness and responded to methylprednisolone therapy, and significant reduction in size and number of lesions was noted in follow-up MRI after 6 months. The patient had no recurrence of illness till 6 years of follow-up. There is no consensus about the treatment of BCS; corticosteroid has been used as a mainstay of treatment. The role of treatment options of MS has not been evaluated in BCS because of the rarity of the disease and different pathophysiological basis. However, beneficial effect of plasmapheresis, interferon beta-1a, mitoxantrone, intravenous immunoglobulin, and azathioprine has been reported in the isolated case report.[3457]
This case report highlights that BCS may be diagnosed on the basis of characteristic MRI findings, and early corticosteroid therapy may be helpful.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Mr. Rakesh Kumar Nigam and Mr. Shakti Kumar for secretarial help.
REFERENCES
- Balo's concentric sclerosis in a 4-year-old Japanese infant. Brain Dev. 1998;20:250-2.
- [Google Scholar]
- Multifocal Balo's concentric sclerosis in a 10-year-old German boy. Neuropediatrics. 2012;43:PS17-09.
- [Google Scholar]
- Balo concentric sclerosis in children: A case series. J Child Neurol. 2014;29:603-7.
- [Google Scholar]
- Pitfalls in the diagnosis of a tumefactive demyelinating lesion: A case report. J Med Case Rep. 2011;5:217.
- [Google Scholar]
- Baló's concentric sclerosis: Clinical and radiologic features of five cases. AJNR Am J Neuroradiol. 2001;22:1362-7.
- [Google Scholar]
- Serial proton MR spectroscopy and diffusion tensor imaging in infantile Balo's concentric sclerosis. Neuroradiology. 2009;51:113-21.
- [Google Scholar]
- CADASIL mutation and Balo concentric sclerosis: A link between demyelination and ischemia? Neurology. 2012;78:221-3.
- [Google Scholar]
- Tissue preconditioning may explain concentric lesions in Baló's type of multiple sclerosis. Brain. 2005;128(Pt 5):979-87.
- [Google Scholar]
- Baló's concentric sclerosis presenting as a stroke-like syndrome. Nat Clin Pract Neurol. 2007;3:349-54.
- [Google Scholar]






