Translate this page into:
Magnetic resonance imaging brain findings in a case of aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder, presenting with intractable vomiting and hiccups
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Neuromyelitisoptica (NMO) and multiple sclerosis (MS) were once considered to be differing manifestation of same auto immune disease, NMO predominantly involving the optic nerve and cord. Now with discovery of NMO antibody the concept has changed and a spectrum of disorders with lesions in brain has been identified. Occasionally, brain may be the first or the only site of involvement in these disorders hence it is essential to be aware of this spectrum. The brain lesions in NMO/NMOSD may be located in characteristic regions and present with symptoms mimicking non neurological disease. We herein present a case of an adult female who was admitted with intractable vomiting and hiccups; subsequently on MRI brain found to have very tiny demyelinating foci in Area Postrema.
Keywords
Aquaporin-4 antibody
area postrema
longitudinally extensive spinal cord lesions
magnetic resonance imaging
multiple sclerosis
neuromyelitis optica
optic neuritis
Introduction
Neuromyelitis optica (NMO) is a complex severe demyelinating disorder of central nervous system that was primarily considered to be characterized by optic neuritis (ON) and myelitis. NMO was considered a disease without brain involvement, which was part of major diagnostic criteria proposed by Wingerchuk et al. in 1999.[1] Ever since the discovery of NMO-IgG/anti-aquaporin-4 (AQP-4) antibody in a proportion of patients with clinically proven NMO and in patients with recurrent, isolated, longitudinally extensive spinal cord lesions (LECS) or ON as well as patients with LECS or ON associated with systemic autoimmune disease or with brain lesions, it has resulted in the identification of a spectrum of disorders called “NMO spectrum disorders” (NMOSD). Although positivity of AQP-4 antibody and presence of brain lesions have been introduced in the revised NMO criteria, the presence of ON and LECS is still mandatory [Table 1].[2] In addition, a separate spectrum for AQP-4 antibody positive patients not fitting into the NMO clinical criteria has been proposed [Table 2].[3]
Isolated and asymptomatic brain findings are now increasingly identified at presentation in these cases; hence, it is imperative to be aware of these brain lesions which might be specific enough to prompt correct diagnosis. In addition, as the symptoms may be dramatic and may not be neurological, we need to be aware of this entity. We herein present a case of an adult woman who had a past history of ON and presented with recent onset hiccups and intractable vomiting.
Case Report
A 45-year-old woman came to the emergency department of our hospital with a history of intractable vomiting and hiccups for 2 days. She was admitted and extensively evaluated in the form of blood investigations and endoscopy. However, the patient was negative for all the work-ups. On enquiry, she gave a vague history of loss of vision in the left eye which improved gradually after taking some medication. She was then sent for neurology opinion and thence referred for magnetic resonance imaging.
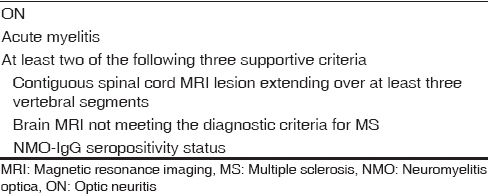
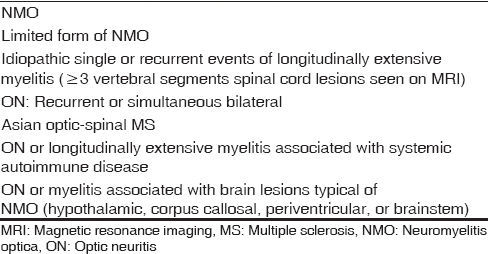
Small hyperintense specks were seen symmetrically in axial fluid attenuation inversion recovery images in the dorsal aspect of the upper medulla at the caudal end of the fourth ventricle (area postrema) [Figure 1]. On sagittal T2-weighted images, thin streak-like hyperintensity was seen [Figure 2]. No restriction of diffusion or definite contrast enhancement was noted [Figures 3 and 4]. In addition, small hyperintense foci were seen in bilateral frontal lobes.
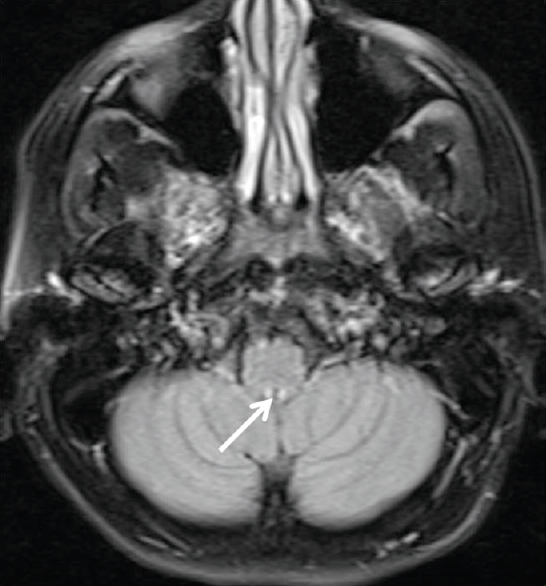
- Fluid attenuation inversion recovery axial image at the level of upper medulla shows tiny hyperintense foci in the dorsal aspect of medulla at the caudal end of fourth ventricle - Area Postrema (arrows)
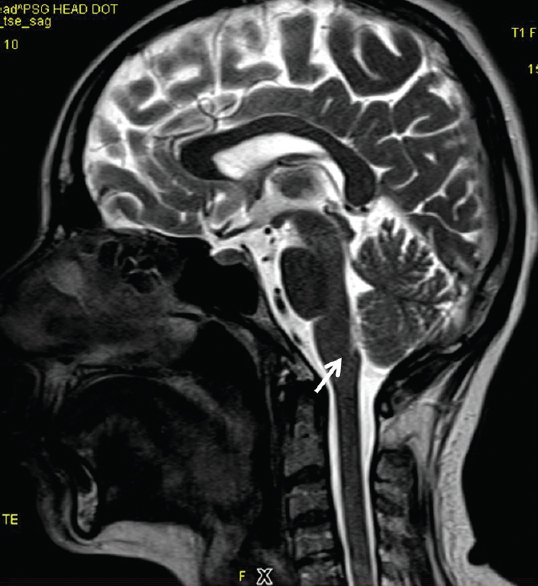
- Mid sagittal T2-weighted image shows faint hyperintense linear streak in the dorsal aspect of upper medulla near the caudal end of fourth ventricle (arrow)
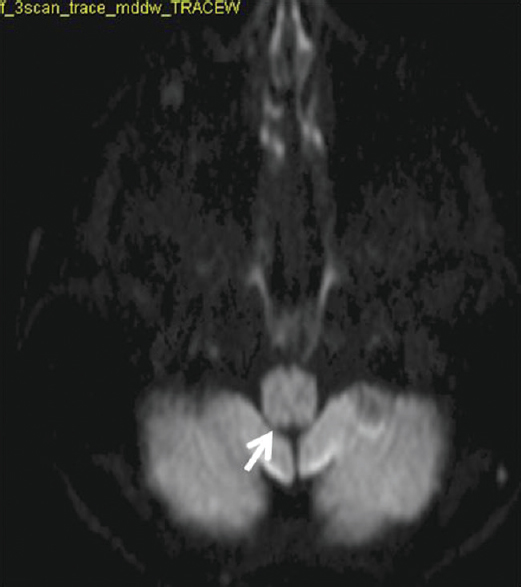
- Diffusion weighted image shows no restriction
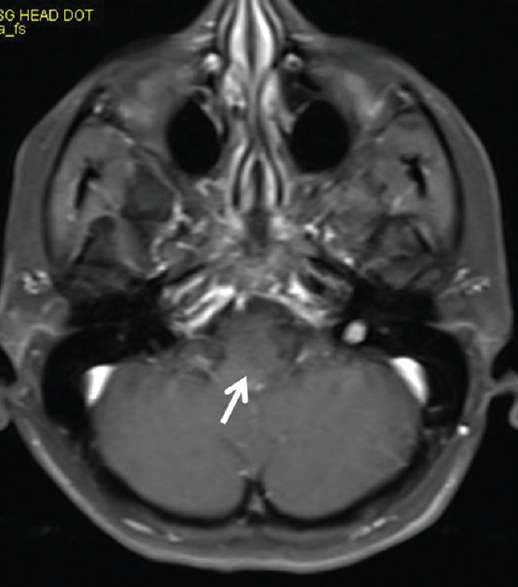
- No enhancement seen on post contrast scan
These lesions were so tiny and nondescript that we had missed them on first analysis, however on review, we came to a conclusion that these indeed could be lesions related to NMOSD. The history of vision loss and recovery which could be suggestive of ON also proved to be corroborative. Anti-NMO antibody analysis gave positive results. As the patient cannot be classified as NMO, at present, due to lack of cord symptoms, she definitely fits the criteria for NMOSD. Currently, the patient is on steroids, and her symptoms have improved significantly.
Discussion
NMO was long regarded as a disease of optic nerve and cord. Lack of brain involvement was traditionally one of the major supportive diagnostic criteria; however, later it was established that asymptomatic brain abnormalities may be seen in up to 60% of the patients with clinically defined NMO. A number of studies have identified a defined spectrum of brain lesion types in up to 93% of the patients with NMO- and AQP-4 antibody-positive NMOSD.[456] Now, with identification of NMOSD, as it is increasingly recognized that some patients present with brain symptoms as their first manifestation or develop recurrent brain symptoms without ON or myelitis, these brain abnormalities have been called to clinicians’ and radiologists’ attention.[78] Nonspecific punctate or small (3 mm) lesions in the deep or, less commonly, subcortical white matter are frequent[46] and usually asymptomatic.
Specific lesion types that are helpful in the radiologic diagnosis of NMO/NMOSD are as follows:[45]
-
Typical lesions in the peri-ependymal regions surrounding the third ventricle, cerebral aqueduct, and fourth ventricle, wherein the AQP-4 antigen is in abundance.[9] Such lesions within the thalamus and hypothalamus may present with a variety of symptoms such as syndrome of inappropriate antidiuretic hormone secretion, narcolepsy, behavioral change, and hypothermia, hypotension, hypersomnia, and obesity.[45] Brain stem lesions can result in a variety of bulbar symptoms, among which intractable hiccups, nausea, and vomiting are relatively specific for NMO and attributable to the involvement of the area postrema and the nucleus tractus solitarius
Our patient had similar symptoms attributable to tiny foci of demyelination in area postrema
-
Periependymal/periventricular and corpus callosum lesions situated in proximity to the lateral ventricles, which need to be differentiated from multiple sclerosis (MS) plaques
-
Unilateral or bilateral, longitudinally extensive lesions involving the corticospinal tract – especially seen in AQP-4 antibody-positive Korean population
-
Extensive hemispheric lesions: Extensive and confluent hemispheric white matter lesions are tumefactive (>3 cm in longest diameter) or configured as long spindle-like or radial-shaped signal changes following white matter tracts such as the longitudinal fasciculus
-
Cortical involvement and leptomeningeal enhancement has been described, but it is very rare.
In addition, these lesions have to be differentiated from MS as in about 5% of the patients with MS may also have the NMO antibody and a subgroup of a NMO patients may be negative for the antibody. More importantly, some of the treatment options of MS may be useless and may actually worsen NMO. Apart from clinical presentations which are usually more fulminant in NMO, initial involvement of cord and optic nerves also point to the diagnosis. Different patterns of spinal cord involvement are noted in the two diseases, long segment cord involvement in NMO (>3 segments) while short segment involvement is seen in MS. Peripheral brain lesions are more common in MS than NMO.[4] Periventricular lesions can be seen in both MS and NMO; however, typical MS, lesions are discrete, ovoid, perpendicular to the ventricles, and perivenular extended (e.g., Dawson's fingers), while NMOSD lesions are located immediately next to the lateral ventricle, following the ependymal lining in disseminated pattern and are often edematous and heterogeneous.[410]
In our case, the patient had a past history of ON, and in the current episode, presented with non-neurological symptoms. The lesions were almost imperceptible. Only on review, the brain lesions could be identified. Thus, emphasizing that it is important to know the brain manifestations of NMO/NMOSD as these may be the first manifestations of this disease and though many findings may be nonspecific, their characteristic locations and configurations are helpful. A thorough understanding of diverse brain manifestations is now crucial for early and correct diagnosis of NMOSD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107-14.
- [Google Scholar]
- Brain abnormalities in neuromyelitis optica spectrum disorder. Mult Scler Int 2012 2012:735486.
- [Google Scholar]
- Brain magnetic resonance imaging abnormalities in neuromyelitis optica. Acta Neurol Scand. 2008;118:218-25.
- [Google Scholar]
- Brain magnetic resonance imaging findings in relapsing neuromyelitis optica. Mult Scler. 2007;13:186-92.
- [Google Scholar]
- Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2011;17:1107-12.
- [Google Scholar]
- Central nervous system aquaporin-4 autoimmunity presenting with an isolated cerebral abnormality. Mult Scler. 2012;18:1340-3.
- [Google Scholar]
- Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964-8.
- [Google Scholar]
- Occurrence of acute large and edematous callosal lesions in neuromyelitis optica. Mult Scler. 2009;15:695-700.
- [Google Scholar]






