Translate this page into:
Leprosy elimination: A myth busted
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Leprosy is mainly a chronic infectious disease caused by Mycobacterium leprae. The disease mainly affects the skin, the peripheral nerves, mucosa of the upper respiratory tract and eyes. Though the target of leprosy elimination was achieved at national level in 2006 even then a large proportion of leprosy cases reported globally still constitute from India.
Aim and Objective:
To study the clinico-epidemiological profile of new cases of leprosy in a rural tertiary hospital.
Materials and Methods:
Thirty-five newly diagnosed cases of leprosy presented in out-patient/admitted in the department of Dermatology, Venereology and Leprosy (between September 2012 and August 2013) were included in the study. Detailed history regarding leprosy, deformity, sensory loss, skin smear for AFB and histopathological examination were done in every patient.
Results:
The incidence was more in age group of 20 to 39 years (48.57%) and 40 to 59 years (37.14%). 68.57% were males. 48.57% cases were found to have facial deformity and ear lobe thickening was found to be pre-dominant form of facial deformity. Ulnar (88.87%) and common peroneal nerve (34.28%) were the most commonly involved nerves. The split skin smear examination was found to be positive in 27 out of 35 cases. On histopathological examination 10 patients (28.57%) were of lepromatous pole (LL), 4 (11.43%) were of indeterminate, 6 (17.14%) were of tuberculoid type (TT), 4 BT (11.4%) and 1 BL type (2.8%).
Conclusions:
This study helps in concluding that leprosy is still not eliminated. Active surveillance is still needed to detect the sub-clinical cases and undiagnosed cases.
Keywords
Deformity
elimination
subclinical cases
Introduction
Leprosy has afflicted humanity since time immemorial. It has left behind a terrifying image in history and human memory-of mutilation, rejection and exclusion from society. Leprosy has struck fear into human beings for thousands of years, and was well recognized in the oldest civilizations of China, Egypt and India.
Leprosy is mainly a chronic infectious disease caused by Mycobacterium leprae. The disease mainly affects the skin, the peripheral nerves, mucosa of the upper respiratory tract and eyes.
There are many countries in Asia, Africa and Latin America with a significant number of leprosy cases. It is estimated that there are between one and two million people visibly and irreversibly disabled due to past and present leprosy who require to be cared for by the community in which they live.
Leprosy still remains an important cause of disability even after adopting the resolution by World Health Assembly in 1991 to “eliminate leprosy as a public health problem by year 2000.”[1] It was the availability of effective multidrug treatment that led to the thought of leprosy elimination despite little understanding of its epidemiology.[23] The combination of biological and epidemiological evidence suggests that the leprosy cannot be eliminated by multidrug therapy alone as the microbiology of leprosy is still not fully elucidated. Leprosy should be grouped under the chronic stable diseases that are being successfully controlled.[4] Though the target of leprosy elimination was achieved at national level in 2006 even then a large proportion of leprosy cases reported globally still constitute from India. In many countries the proportion of MB Leprosy cases among new cases remains still high like, for example, in Democratic Republic of Congo (72%), Indonesia (81%), Cuba (83%) and Kenya (99%).[5] Not only the indicator of active transmission, that is, proportion of children among new cases remains high (>20%) in countries like Liberia, Dominican Republic, Indonesia but also the trend shows increases in Nepal and Sudan up to 5% in the past few years and continues to remain high in India.[56] The indicator of late detection, that is, the proportion of new cases with grade-2 disabilities has also shown at higher levels in Madagascar, Sudan, and China. Out of the 17 countries, in 9 countries including India the trend is rising (from 2.2% in 2006 to 3.1% in 2010).[5] In 2011, of the total 219,075 new leprosy cases reported globally, 58.1% were detected only in India.[7] According to WHO Weekly Epidemiological Record 2013 the South-east Asian region accounts for 71% of new cases detected worldwide. Out of global total 232,857, new patients 134,752 have been detected in India in 2012 according to WER 2013.[8]
According to WHO Weekly Epidemiological Record 2013 the number of cases of leprosy (rate per 100 000 population) with grade-2 disabilities among new cases reported is 0.43. In 2009, of the 244,796 new cases reported globally, 133,717 were detected in India. Among them more than 10% were children which strongly indicate that active transmission is occurring.[9]
Objectives
To study the clinico-epidemiological profile of new cases of leprosy in a rural tertiary hospital.
Materials and Methods
The present study was conducted in the department of dermatology in a rural medical college in central India. The study was done from September 2012 to August 2013. Diagnosed leprosy patients either coming to out-patient department (OPD) or in-patient department (IPD) were included in the study. Thirty-five newly diagnosed cases of leprosy were detected during this period. Informed consent was taken from all the patients and only those patients providing consent were included in the study.
A structured pre-designed, pre-tested questionnaire was used to collect data related to socio-demographic profile and to record the findings or clinical examination and laboratory investigations.
After obtaining informed consent, brief history regarding the onset of symptoms and previous treatment taken, if any were obtained followed by thorough clinical examination of all the cases. After a thorough cutaneous and neural assessment, routine hematological investigations such as complete blood counts, blood sugar, and erythrocyte sedimentation rate were done followed by skin slit smear for acid-fast bacilli and histopathology.
All patients were diagnosed in the same way: first they were checked for the presence of skin and/or nerve lesions consistent with leprosy. Then slit skin smear examination was performed to demonstrate the presence of acid-fast bacilli. Subsequently patients were further classified into multi-bacillary (MB) or pauci bacillary (PB) patients, using the WHO classification based on number of lesion and skin smear positivity for AFB.[10] MB was defined as smear positive (with any number of lesions) or smear negative with more than five lesions, while the PB classification was assigned to smear-negative patients that had a maximum of five lesions. Biopsy was also done for histopathological examination. Patients are also examined for deformity and graded according to WHO classification of deformities of hand and foot.[11]
WHO Grading of hands and feet deformity
Grade 0: No anesthesia/no visibledeformity
Grade 1: Anesthesia present but no visible deformity
Grade 2: Visible deformity/damage.
The collected data were encoded and entered electronically in computer using Epi info version 3.5.4 (CDC, Atlanta) and statistical analysis was also performed using the same software.
Results
The basic socio-demographic profile of the patients is depicted in Table 1 which shows that out of 35 patients, 68.57% were males and 31.43% were females. 48.57% patients were from the age group of 20-39 years and 48.57% patients were farmers. 68.57% belong to upper middle class according to Kuppu-Swami's classification.[12] In 34.29% of patients history of overcrowding was present.
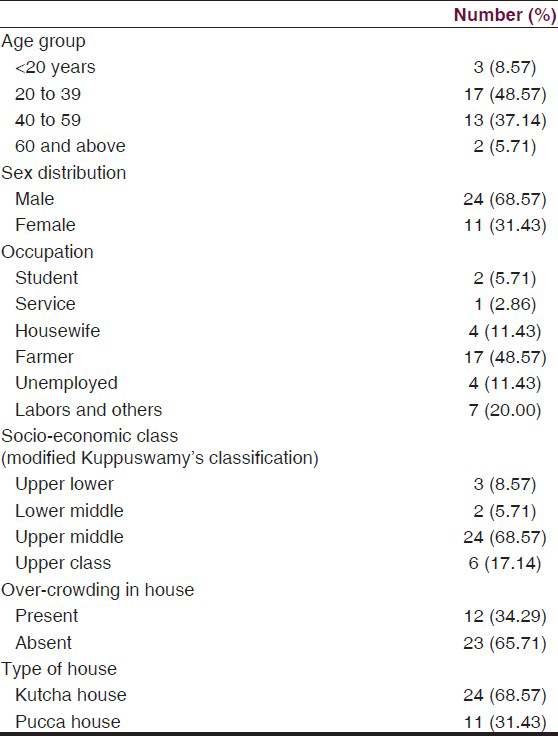
The association of systemic disease is depicted in Table 2. 8.57% patients had a history of systemic disease which include diabetes (5.71%) and HIV (2.86%). 2.86% patients had a positive family history of Hansen's disease.

45.7% patients had Grade 1 deformity and 2.9% had grade 2 deformity [Table 3]. Table 4 shows the facial deformities prevalence which were present in the form of depressed nasal bridge (8.6%), ciliary madarosis (2.9%) and ear lobe thickening (37.1%) [Table 4]. Table 5 shows that the deformities are mostly present in the age group of 20-39.


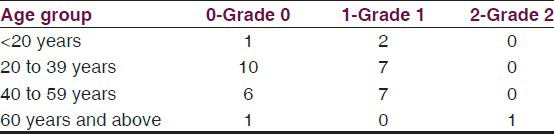
Table 6 is showing the relation of nerve involvement in leprosy. In 31 (bilateral ulnar nerve in 74.3% and unilateral in 14.3%) patients ulnar nerve was found to be thickened followed by common peroneal nerve which was thickened in 12 patients (34.28%). Median nerve was involved in 2.9%. In 20% patients of the patients posterior tibial nerve was thickened.

Histogram in Figure 1 shows that eight patients were found to be negative for split skin AFB while the remaining patients were positive for SSS (Slit Skin Smear) for AFB. The value for SSS for AFB was between 5.1 and 6 for four patients which indicates a very high bacillary load.
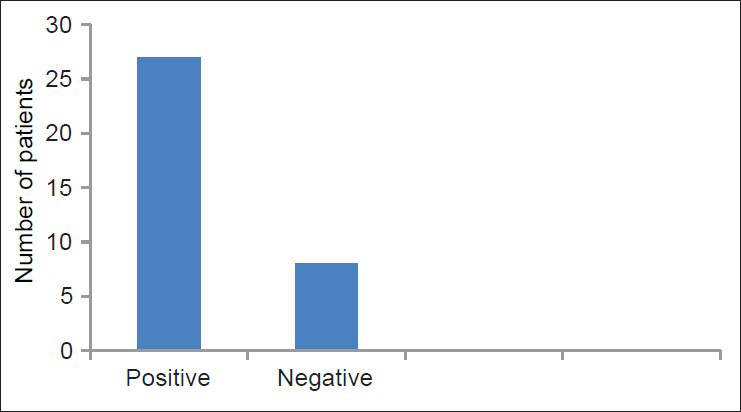
- Results of split skin smear (SSS) for acid fast bacilli in leprosy
The pie chart in Figure 2 shows the histopathological classification of leprosy. Ten patients (28.57%) were of lepromatous pole (LL-lepromatous leprosy), 4 (11.43%) were of indeterminate, 6 (17.14%) were of tuberculoid type (TT), 4 BT (borderline tuberculoid) (11.4%) and 1 BL (borderline lepromatous) type (2.8).
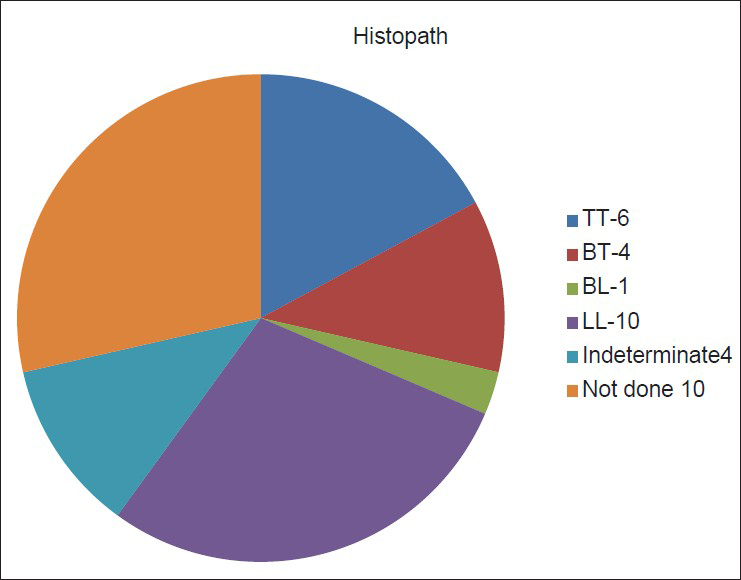
- Pie chart showing histopathology pattern in leprosy. (TT = tuberculoid leprosy, BT = borderline tuberculoid, BL = borderline lepromatous, LL = lepromatous leprosy, I = indeterminate)
Discussion
The goal of WHO for the elimination of leprosy has given a boost in this direction but has it been achieved? Global alliance has played a crucial role by publishing its evaluation report and recommended that the World Health Assembly should pass a clear cut resolution to the world community that still the mankind could not be get rid of from the clutches of leprosy as it is not eliminated.[13141516] There may be a political agenda, aspiration of the people but the medical and scientific disciplines have not acquired enough base and ground to eradicate this disease.[17]
The present study also indicated high load of undiagnosed cases in the community. Leprosy seems to be affecting people across all age group although the incidence was more in the age group of 20 to 39 years (48.57%) and 40 to 59 years (37.14%). Leprosy is known to have male preponderance; in our study too the majority of the cases were of males (68.57%). Contrary to prior evidences, in our study the maximum number (68.57%) of leprosy cases were reported from upper middle socio-economic group. Presence of over-crowding in the house was not found be important contributor in incidence of leprosy in our study. Associated medical history or family history of Hansen's disease was not associated with higher incidence of leprosy according to the present study [Table 2].
In the present study, 48.57% cases were found to have facial deformity and ear lobe thickening was found to be pre-dominant form of facial deformity [Table 4]. Majority of the cases presented with grade 0 deformity [Table 5]. The present study also strengthens the evidence that the ulnar (88.87%) and common peroneal nerves (34.28%) involvement is most common in leprosy cases [Table 6]. One important finding in this study is that majority of the patients are MB type and on histological examination the most common pattern was found to be lepromatous pattern. While in other studies BT was found to be the most prevalent type of leprosy in India.[18] The split skin smear examination was found to be positive in 27 out of 35 cases, that is, 77.1% [Figure 1].
The delivery of better health care system and management of better quality leprosy services can shoulder to a great extent while good referral system can bring a revolutionary faith in the minds of the masses. We can achieve a major breakthrough results by reducing the deformity through early detection, self care, physiotherapy and reconstructive surgery and developing sound surveillance systems.
The political will power and vision and mission of the government can prove to be a guiding star and as well as devising strategies for eradication, elimination and prevention of this disease by the scientific and techno managerial professional community should go hand in hand in perfect harmony with the government.
Conclusion
The clinical, bacteriological, and histopathological characteristics of newly detected cases evidently indicate the grave nature of the problem. This study helps in concluding that leprosy is still not eliminated. Lepromatous type was found to be the most common pattern. Active surveillance is still needed to detect the sub clinical cases and undiagnosed cases.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Elimination of Leprosy: Resolution of the 44th World Health Assembly. Geneva: World Health Organization; 1991.
- [Google Scholar]
- World Health Organziation Expert Committee on Leprosy. Seventh Report. World Health Organ Tech Rep Ser. 1998;874:20.
- [Google Scholar]
- Epidemiology and control of leprosy: A review of progress over the last 30 years. Trans R Soc Trop Med Hyg. 1993;87:515-7.
- [Google Scholar]
- “Neglected” diseases but unrecognized successes-challenges and opportunities for infectious disease control. Lancet. 2004;364:380-3.
- [Google Scholar]
- Childhood leprosy in a tertiary-care hospital in Delhi, India: A reappraisal in the post-elimination era. Lepr Rev. 2011;82:259-69.
- [Google Scholar]
- Leprosy in post-elimination era in India: Difficult journey ahead. Indian J Dermatol. 2013;58:443-6.
- [Google Scholar]
- Rapid quantitative serological test for detection of infection with Mycobacterium leprae, the causative agent of leprosy. J Clin Microbiol. 2014;52:613-9.
- [Google Scholar]
- Kuppuswamy's socioeconomic status scale-a revision. Indian J Pediatr. 2003;70:273-4.
- [Google Scholar]
- Leprosy: Too complex a disease for a simple elimination paradigm. Bull World Health Organ. 2005;83:230-5.
- [Google Scholar]
- An evaluation of GAEL, the Global Alliance for the Elimination of Leprosy. Lepr Rev. 2004;75:208-13.
- [Google Scholar]
- Comments on the report entitled ‘Independent evaluation of the Global Alliance for the Elimination of Leprosy’. Lepr Rev. 2004;75:217-20.
- [Google Scholar]
- Clinical, bacteriological, and histopathological characteristics of newly detected children with leprosy: A population based study in a defined rural and urban area of Maharashtra, Western India. Indian J Dermatol Venereol Leprol. 2013;79:512-7.
- [Google Scholar]






