Translate this page into:
Lead encephalopathy in adults
Address for correspondence: Dr. B. Vengamma, Department of Neurology, Sri Venkateswara Institute of Medical Sciences, Tirupati - 517 507, Andhra Pradesh, India. E-mail: bvengamma@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Lead poisoning is a common occupational health hazard in developing countries. We report the varied clinical presentation, diagnostic and management issues in two adult patients with lead encephalopathy. Both patients worked in a battery manufacturing unit. Both patients presented with seizures and one patient also complained of abdominal colic and vomiting. Both were anemic and a lead line was present. Blood lead level in both the patients was greater than 25 µg/dl. Magnetic resonance imaging of brain revealed bilateral symmetric involvement of the thalamus, lentiform nucleus in both patients and also the external capsules, sub-cortical white matter in one patient. All these changes, seen as hyperintensities in T2-weighted images suggested demyelination. They were advised avoidance of further exposure to lead and were treated with anti-epileptics; one patient also received D-penicillamine. They improved well on follow-up. Lead encephalopathy is an uncommon but important manifestation of lead toxicity in adults.
Keywords
Chelator
Chronic lead poisoning
lead battery workers
lead encephalopathy
Introduction
Most common causes of lead poisoning in adults are occupational exposure and traditional medicine usage.[1] In such patients encephalopathy is an uncommon presentation.[2] We report two rare cases of chronic lead encephalopathy that occurred in battery manufacturing factory workers.
Case Reports
Case 1
A 46-year-old male presented with cluster attacks of left focal motor seizures with secondary generalization over 4 days prior to examination. General physical examination revealed pallor and dark blue lead lines present on the gums [Figure 1a]. Vital signs were normal. Neurological examination was normal.
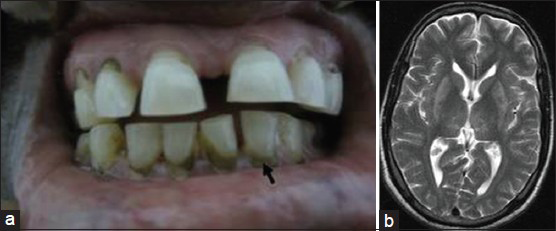
- Clinical photograph shows dark blue lead lines present on the gums (a). MRI brain shows bilateral symmetric involvement of the thalamus and lentiform nucleus with the hyper intensities in T2-weighted axial images suggesting sub-cortical white matter toxic demyelination (b)
Laboratory investigations revealed hemoglobin 8.1 g/dl and hypochromic microcytic anemia without evidence of erythrocytic basophilic stippling. Blood lead level measured by graphite furnace atomic absorption spectrometry (GFAAS) with the Zeeman correction method was 38 µg/dl. Ultrasonography of the abdomen showed grade I renal parenchymal changes. Electroencephalogram (EEG) and nerve conduction studies (NCS) were normal. Magnetic resonance imaging (MRI) of brain showed bilateral symmetric involvement of the thalamus and lentiform nucleus with hyper intensities in T2-weighted axial sequences [Figure 1b]. The patient was advised avoidance of further exposure to lead and was treated with carbamazepine (10 mg/kg). He had no recurrence of seizures for 18 months of follow-up.
Case 2
Another adult patient, a 38-year-old male, presented with convulsive status epilepticus (generalized tonic and clonic seizure). During the past 4 years, he had recurrent episodes of severe abdominal colic associated with vomiting. General physical examination revealed pallor and a lead line was also evident [Figure 2a]. Vital signs were normal except for blood pressure 170/90 mmHg. Neurological examination was unremarkable.

- Clinical photograph shows dark blue lead lines present on the gums (a). MRI brain shows bilateral symmetric involvement of the thalamus, lentiform nucleus, sub-cortical white matter and external capsule in T2-weighted axial sequences, with the hyper intensities suggesting toxic demyelination (b)
Laboratory testing showed hemoglobin 7.7 g/dl and hypochromic microcytic anemia; basophilic stippling was not evident. Blood lead level measured by GFAAS was 97.5 µg/dl. Ultrasonography of the abdomen showed grade I renal parenchymal disease changes. NCS showed motor axonal neuropathy in four limbs and EEG was normal. MRI of the brain showed bilateral symmetric hyperintensities of the thalamus, lentiform nucleus, external capsules and sub-cortical white matter in T2-weighted axial sequences [Figure 2b].
The patient was advised avoidance of further exposure to lead. He was treated with a loading dose of phenytoin (20 mg/kg), followed by a maintenance dose of phenytoin (5 mg/kg/day). He was also treated with D-penicillamine (25 mg/kg/day) for 6 months, and with amlodipine (5 mg/day) for control of hypertension. The patient showed symptomatic improvement during a one year follow-up.
Discussion
Lead poisoning affects multiple organs. Neurological involvement is characterized by brain edema, demyelination of the cerebral and cerebellar white matter and demyelinating peripheral neuropathy.[234] Lead encephalopathy is much rarer in adults than in children.[2] Adults have increased resistance to the development of lead encephalopathy due to the capacity of the mature adult brain to sequester lead away from its mitochondrial site of action within cerebral and cerebellar neurons.[45] Lead acts as a cellular toxin by inhibiting mitochondrial respiration.[5] Lesions in the central nervous system are thought to be the result of vascular injury.[5] Other manifestations include a lead line (Burtonian line) on the gums, hypertension, hypochromic microcytic anemia, punctate basophilic stippling of erythrocytes in some cases, abdominal colic, chronic tubulointerstitial renal disease, lead bands at epiphyses of the long bones, and predisposition to gout.[2]
Both patients presented with seizures, which are due to alterations in blood-brain–barrier integrity or due to inhibition of neurotransmission by gamma-amino butyric acid.[6] One of our patients also presented with abdominal colic, which is due to contraction of the smooth muscle of the intestinal wall in this condition.[2]
Lead lines are caused by the reaction of circulating lead with sulphur ions released by oral bacterial activity, resulting in deposits of lead sulfide in the gums.[2] These lead lines are evident in both of our patients. Both presented with anemia which is due to inhibition of pyrimidine 5’-nucleotidase.[2] Our patients have no evidence of basophilic stippling of peripheral blood erythrocytes, which is more pronounced in the bone marrow than in the peripheral blood.[2] Hypertension in one of our patients could have been a consequence of lead poisoning through direct effects on the cardiovascular system or from the development of interstitial nephritis.[2]
The Centre for Disease Control and Prevention, Atlanta, USA, has defined elevated blood lead levels as a value more than 25 µg/dl of whole blood in adults; for young children the corresponding value has been defined as 5 µg/dl.[7] Any lead in blood is of concern and requires monitoring. Ultrasonography of the abdomen shows grade I renal parenchymal changes in both cases suggestive of renal involvement. Even though both of our patients lack symptomatic peripheral neuropathy, NCS reveal evidence of motor axonal neuropathy in one patient, which is due to Schwann cell destruction followed by demyelination and axonal atrophy.[23] In lead poisoning, bilateral thalamic hyperintensities have been reported on T2-weighted MRI brain images.[4] In another study from India, bilateral symmetric involvement of the occipital lobe affecting predominantly the gray-white matter junction and the sub-cortical white matter is reported in lead poisoning.[8] In our study, MRI brain shows bilateral symmetric involvement of the thalamus and lentiform nucleus with hyperintensities in T2-weighted axial images suggesting sub-cortical toxic demyelination in both patients. In one of our patients, evidence of demyelination is also found in the external capsules of the cerebrum.
Our patients have chronic, severe lead toxicity according to the criteria of the Occupational Lead Poisoning Prevention Program.[9] These clinical observations are substantiated by whole blood lead level measurements, which are greater than 25 µg/dl in both of our patients.[7]
We follow revised treatment guidelines laid down by Association of Occupational and Environmental Health Clinics.[10] Both patients have been advised to avoid further lead exposure. Both received treatment with anti-epileptic drugs and the chelating agent D-penicillamine was initiated for one patient.[10] During follow-up there has been no seizure recurrence in either patient and there is good control of relief from abdominal pain and good blood pressure control.
Lead encephalopathy is an uncommon manifestation of lead toxicity in adults. In India, it is commonly seen in workers engaged in battery manufacturing units and those who use traditional medicines.[1] Avoidance of further exposure to lead is the main modality of treatment.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Lead-induced peripheral neuropathy following Ayurvedic medication. Indian J Med Sci. 2009;63:408-10.
- [Google Scholar]
- Cerebellar ataxia due to lead encephalopathy in an adult. J Neurol Neurosurg Psychiatry. 1998;65:797.
- [Google Scholar]
- Maturation of resistance to lead encephalopathy: Cellular and sub cellular mechanisms. Neurotoxicology. 1984;5:97-124.
- [Google Scholar]
- Lead, GABA, and seizures: Effects of subencephalopathic lead exposure on seizure sensitivity and GABAergic function. Environ Res. 1979;19:371-82.
- [Google Scholar]
- Centers for Disease Control and Prevention. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention”. Available from: http://www.cdc.gov/nceh/lead/ACCLPP/CDCResponse Lead Exposure Recs.pdf. Article
- [Google Scholar]
- Pre- and post-treatment MR imaging findings in lead encephalopathy. AJNR Am J Neuroradiol. 2006;27:902-3.
- [Google Scholar]
- Occupational Lead Poisoning Prevention Program (OLPPP) Available from: http://www.cdphca.gov/programs/olpp/Pages/default.aspx
- [Google Scholar]
- Association of Occupational and Environmental Health Clinics. Medical management guidelines for lead. exposed adult. Revised 04/24/2007. Available from: http://www.aoec.org/documents/positions/mmg final.pdf
- [Google Scholar]






