Translate this page into:
Lack of habituation of visual-evoked potential in the interictal period is not a consistent neurophysiological marker of migraine: A cross-sectional analytical study
*Corresponding author: Ramkumar Sugumaran, Department of Neurology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. ramkumar.sugumaran@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rani A, Sugumaran R, Narayan SK. Lack of habituation of visual-evoked potential in the interictal period is not a consistent neurophysiological marker of migraine: A cross-sectional analytical study. J Neurosci Rural Pract. 2024;15:536-44. doi: 10.25259/JNRP_33_2024
Abstract
Objectives:
Migraine is a frequent incapacitating neurovascular illness characterized by severe headache bouts. Individuals suffering from migraine appear to process auditory and visual information differently from those without migraine. The visual-evoked potential (VEP) is a commonly used standardized test to measure excitability in the occipital cortex. Patients with migraine exhibit amplification rather than habituation of stimulus-induced brain responses, between attacks. Our objective is to compare the amplitude of P100 and the latencies of N75, P100, and N145 (N and P represent negative and positive peaks, respectively, with average latency being subscripted with the alphabet) in the fourth block between migraine patients and controls and to determine the various clinical factors associated with the P100 mean amplitude and latency differences between the first and the fourth block in migraine patients.
Materials and Methods:
The study compared 20 migraine patients (with or without aura) and 20 apparently healthy subjects with no history of migraines or secondary headaches, focusing on the habituation of the VEP. Four blocks of 200 responses were recorded during the headache-free period, and the latencies and amplitudes of N75, P100, and N145 components were analyzed.
Results:
There was a statistically significant (P < 0.05) decrement in the P100 amplitude in the fourth block when compared to the first block in both eyes in the controls as well as migraine patients. In addition, there was no statistically significant difference between controls and migraine sufferers in the P100 amplitude of the fourth block in either eye. The N145 latency in the fourth block was shorter in both eyes in migraine patients compared to controls (P < 0.05). The mean P100 amplitude difference between the first and fourth block correlated negatively with age and positively with headache frequency, while there was a moderate negative correlation with headache duration. The mean P100 latency difference between the first and fourth block correlated positively with age and negatively with headache frequency, while there was a moderate positive correlation with headache duration.
Conclusion:
In our study, VEP habituation was not lacking in migraine patients which means that habituation of the P100 wave was noted in migraineurs. The VEP reveals neurological changes due to ischemia injury or neurotransmitter imbalances. Migraine alters cortical excitability, but it is unclear if these changes are due to altered excitatory connections, damaged inhibitory networks or subcortical pre-activation. Our findings suggest that at least during the interictal period, lack of habituation cannot be employed as a consistent neurophysiological marker of migraine across laboratories.
Keywords
Migraine
Headache
Visual-evoked potential
Habituation
Potentiation
INTRODUCTION
Migraine is a widespread neurovascular disorder with a high prevalence resulting in socioeconomic, personal, and other repercussions. The International Headache Society classifies migraine headaches as with or without an aura. In 82% of cases, patients experience the visual symptoms of migraineous aura, which include scotoma, a central or paracentral blind spot, stars, zigzags, and flashes of light.[1] According to studies, migraineurs exhibit alterations in their evoked potential even when they are not experiencing headaches.[2] The brain’s sensitivity to migraine attacks appears to be mostly dependent on cortical excitability during migraine interictal periods. Patients with migraines exhibit amplification rather than habituation of stimulus-evoked brain responses between attacks. The main structures involved in the pathogenesis of migraine are the brainstem, trigeminovascular system, and cerebral cortex. It is unclear whether the change in habituation is caused by higher or lower cortical pre-activation levels. Increased or decreased cortical excitability may contribute toward the pathogenesis. Mainly two theories prevail to explain the cortical excitability in migraineurs, which are the theory of hypoexcitability and the theory of hyperexcitability. Authors, who support the idea of decreased pre-activation in migraineurs, emphasize lower initial P100 amplitude during repetitive stimulation[3] while some studies[4] showed the migraineurs being characterized by an increase in neuronal excitability. There has been substantial research into the biochemical and neurophysiological abnormalities that lead to migraine attacks, but the inconspicuous element that causes the disorder, which, if present, should also be visible during a period of pain-free time, is the underlying dysfunction.[5] Visual-evoked potentials (VEPs) are a functional option that smoothens the path of assessment of the visual pathway. Pattern-reversal VEPs can be used to explore the two parallel routes that carry visual information: The luminance and contour processing pathways. These pathways are hypothesized to be differentially affected in migraine.[6] The mass activity of visual cortex neurons is inferred from cortical VEPs. In a previous pattern reversal (PR)- VEP study, during the interictal stage, potentiation substituted the normal habituation pattern in migraineurs.[7]
The visual cortex appears to exhibit habituation of the VEP as a physiological phenomenon when it malfunctions during episodes of migraine. Cortical activity is habituated and potentiated by chemically regulated connections that come from the brainstem and may use transmitters such as serotonin, noradrenaline, dopamine, histamine or acetylcholine. Through the assessment of VEP in migraine patients in between attacks and comparison with normal patients, this study aims to gain insight into the pathophysiology of migraine. The brain’s vulnerability to migraine attacks appears to be mostly determined by the cerebral cortex’s excitability during the interictal state of migraine. Besides, no previous studies have analyzed the various clinical factors associated with the P100 mean amplitude and latency differences between the first and the last block in migraine patients on consecutive VEP stimulations. This will be important in determining the clinical factors that are associated with the lack of VEP habituation in migraine patients. This study helped us to know the cortical excitability by analyzing the habituation of VEP in between attacks, which can be used as an objective measurement of prophylactic treatment response. Furthermore, it can be used to compare the efficacy of various migraine prophylactic drugs, thereby aiding in better treatment outcomes.
MATERIALS AND METHODS
Study setting and design
The study was conducted in the outpatient clinic of the Department of Neurology, after obtaining Institutional Ethics Committee approval (JIP/IEC/2021/0247), and consent was taken from each participant before the study. A total number of 40 participants (20 migraine patients and 20 normal individuals), aged 18–60 years, were recruited for the study. Two groups were studied, namely, the case group and the control group. In the case group, participants were chosen for the study if they had been diagnosed with a migraine headache, with or without aura, according to the third edition of the International Classification of Headache and had no other neurological disorder. Patients must have a headache frequency of <15 episodes per month (episodic migraine). Furthermore, patients must be headache-free for at least one week before the VEP study. Patients with H/O migraine disorders other than episodic migraine, any severe systemic illness such as acute or chronic renal failure, uncontrolled systemic hypertension/DM, malignancy, previous cervical spine surgery or craniotomy, heart disease, psychiatric illness, epilepsy, cerebrovascular diseases, and pregnant and lactating women were excluded from the study. Patients on prophylactic medication and other nonsteroidal anti-inflammatory drugs were also included in the study. Healthy subjects, who had not been diagnosed with migraine headaches with or without aura and with no other secondary causes of headaches, were included in the control group. According to a prior study by Vijayalakshmi et al.,[2] and based on Open epi version 3.1, it was suggested that a sample size of 20 patients was required, with 10 patients in each group, to achieve an 80% power and a 95% confidence interval for comparing migraine patients and controls. This was based on the assumption that there would be a group difference of 2.6 and a standard deviation (SD) of 2.6 in migraine patients and 1.1 in controls in the P100 amplitude in the fourth block. However, since the sample size was small, we included a feasible sample size of 40 with 20 patients in each group.
Instruments used
The VEP test was performed using the Nihon Kohden ENMG/EP measuring system machine. After washing their hair, the patients were advised not to apply any oil or hair spray before the test. Patients with refractory errors were instructed to put on their regular glasses. The subjects were instructed not to move or blink frequently during the VEP test to reduce muscle contraction artifacts from the subject’s eyes and skeletal muscles, which distort the evoked potential waves. The VEP test was conducted in a dark, silent room with the subjects sitting comfortably on a chair.
The Oz (recording electrode), Fz (reference electrode), and FPz (ground electrode) were placed according to the 10–20 placement system. The television set (PR Stimulator) was placed 1 m from the subject’s nasion. The preamplifier was wired up to the electrodes. The low-pass filter was set at 100 Hz and the high-pass filter at 1 Hz with sweep speed, duration, and sensitivity at 50 ms/div, 30 ms, and 5 μV, respectively. The number of epochs was 200, and the amplification ranged from 20,000 to 100,000. The electrode impedance was kept below 3 kΩ. With PR stimuli, a black and white checkerboard with an 80% contrast, and a spatial frequency of 2 cycles per degree was used. The alternating checker-board pattern was utilized as the visual stimuli in the PR stimulation technique, which was binocularly presented on a video screen with a fixation point for full field at >8°. The pattern was 8 × 8 min in size, and the stimulus frequency was 1 Hz. The subjects were instructed to fix their gaze at the center of the screen. The stimulus was given continuously for approximately 12.8–13 min. This 12.8-min time was broken up into four 3.2-min blocks, 200 epochs on average make up each block. The peak latency and peak-to-peak amplitude of both the positive and negative waves were assessed after the response was recorded and displayed on the monitor. The VEP results were interpreted by a person blinded to the diagnosis.
Outcomes
The primary outcome was to compare the amplitude of P100 in the fourth block between migraine patients and controls. The secondary outcomes were, first to compare the latencies of N75, P100, and N145 in the fourth block between migraine patients and controls; secondly, to determine the various clinical factors associated with the P100 mean amplitude and latency differences between the first and the fourth block in migraine patients.
Statistical methods
Demographics were described using mean, SD, frequency, and percentage. We verified data distribution through visual analysis of the Q-Q plot. We found that sample data followed the normal distribution; hence, we adopted parametric tests for comparisons. For comparing continuous data between groups, an independent t-test was performed, and for categorical variables, a Chi-square test was performed. To examine the link between the dependent variable and independent variables in correlation and regression, Pearson’s correlation and multiple regressions were used. P < 0.05 was the threshold for significance. Statistical analysis was performed using data analysis in Microsoft Office 21 and R Studio version 4.2.2.
RESULTS
A total of 40 subjects were included in the study out of which 20 subjects were recruited as controls and 20 subjects with migraine were recruited as cases. The mean age of the patients was 34.4 ± 8.72 years and that of controls was 26.45 ± 5.45. The mean frequency of the headache in migraine patients was 7.7 ± 4.01 episodes per month, and the duration of the headache was 6.65 ± 1.93 h. Baseline characteristics and demographic features were compared between cases and matched controls [Table 1].
| Characteristics | Case group (n=20) | Control group (n=20) | P-value |
|---|---|---|---|
| Age* (in years) | 34.4±8.72 | 26.45±5.45 | 0.004 |
| Gender (%) | |||
| Male | 8 (40) | 12 (60) | 0.134 |
| Female | 12 (60) | 8 (40) | 0.134 |
| Distribution (%) | |||
| Holocranial | 8 (40) | ||
| Hemicranial | 11 (55) | ||
| Occipitonuchal | 1 (5) | ||
| Character (%) | |||
| Throbbing | 16 (80) | ||
| Stabbing | 1 (5) | ||
| Steady | 3 (15) | ||
| Frequency* (episodes/month) | 7.7±4.01 | ||
| Duration* (in years) | 2.005±0.23 | ||
| Timing* (in hours) | 6.65±1.93 | ||
| Severity (%) | |||
| Mild | 2 (10) | ||
| Moderate | 13 (65) | ||
| Severe | 5 (25) | ||
| Symptoms (%) | |||
| Photophobia | 9 (45) | ||
| Phonophobia | 8 (40) | ||
| Nausea | 11 (55) | ||
| Migraine with aura (%) | 3 (15) | ||
| Migraine without aura (%) | 17 (85) | ||
| Medication (%) | |||
| Prophylactic | 8 (40) | ||
| SOS | 10 (50) | ||
| No medication | 2 (10) |
The amplitude of P100 in the fourth block decreased in both eyes of the controls compared to the first block [Table 2a], which was statistically significant (P = 0.003 [left side]; P = 0.002 [right side]). Similar to this, there was a statistically significant (P < 0.001) drop in the amplitude of P100 in the fourth block compared to the first block in both eyes of the migraine patients [Table 2a]. The N145 latency in the fourth block was shorter in both eyes in migraine patients compared to controls (P = 0.01) [Table 2b]. However, there was no statistically significant difference between migraine patients and controls in the amplitude or latency of P100 in the fourth block in either eye [Tables 2c and d].
| (a) Comparison of P100 amplitude in the control group and migraine patients | |||||
| P100 amplitude (mV) in the control group | |||||
| Side | Pair | N | Mean±SD | t value | P value |
| Left side | 1st block | 20 | 8.041±3.574 | ||
| 4th block | 20 | 7.443±3.176 | 3.32329969 | p=0.003 | |
| Right side | 1st block | 20 | 8.272±3.666 | ||
| 4th block | 20 | 7.595±3.103 | 3.17235365 | P=0.002 | |
| P100 amplitude (mV) in the migraine patients | |||||
| Side | Pair | N | Mean±SD | t value | P value |
| Left side | 1st block | 20 | 8.417±3.403 | ||
| 4th block | 20 | 6.944±3.127 | 5.15 | p<0.001 | |
| Right side | 1st block | 20 | 8.496±3.502 | ||
| 4th block | 20 | 6.822±3.210 | 5.13674493 | p<0.001 | |
| (b) Comparison of N75 and N145 latencies (ms) in the 4thblock between the migraine patients and controls | |||||
| Variables | Side | Group | N | Mean±SD | P value |
| N75 | Left side | Migraine patients | 20 | 71.425±6.983 | |
| Controls | 20 | 70.725±5.5712 | p=0.728 | ||
| Right side | Migraine patients | 20 | 71.350±6.8712 | ||
| Controls | 20 | 69.100±5.3376 | p=0.255 | ||
| N145 | Left side | Migraine patients | 20 | 135.300±9.8052 | |
| Controls | 20 | 147.175±19.7406 | p<0.01 | ||
| Right side | Migraine patients | 20 | 134.700±11.9950 | ||
| Controls | 20 | 148.600±18.4124 | p<0.01 | ||
| (c) Comparison of P100 amplitude (mV) in the 4thblock between the migraine patients and controls | |||||
| Side | Group | N | Mean±SD | t value | P value |
| Left side | Migraine patients | 20 | 6.944±3.127 | ||
| Controls | 20 | 7.443±3.176 | -0.5344568 | p=0.59 | |
| Right side | Migraine patients | 20 | 6.822±3.210 | ||
| Controls | 20 | 7.595±3.103 | -0.796752 | p=0.43 | |
| (d) Comparison of P100 latencies (ms) in the 4thblock between the migraine patients and controls | |||||
| Side | Group | N | Mean±SD | P value | |
| Left side | Migraine patients | 20 | 103.100±8.202 | ||
| Controls | 20 | 104.175±7.765 | p=0.67 | ||
| Right side | Migraine patients | 20 | 104.600±8.144 | ||
| Controls | 20 | 100.275±22.829 | p=0.43 | ||
| (e) Amplitude of P100wave (mV) from 1stblock to 4thblock | |||||
| Side | Mean values | ||||
| Controls | Migraine patients | ||||
| Left side | 1st block | 8.1 | 8.4 | ||
| 2nd block | 7.7 | 7.7 | |||
| 3rd block | 7.6 | 8.1 | |||
| 4th block | 7.4 | 6.9 | |||
| Right side | 1st block | 8.2 | 8.4 | ||
| 2nd block | 7.8 | 7.6 | |||
| 3rd block | 7.7 | 7.6 | |||
| 4th block | 7.5 | 6.8 | |||
SD: Standard deviation
The amplitude decreases progressively from block one to block four [Table 2e] in both the control and migraine patients, while there is no significant difference between the two groups.
Multiple regressions were run to predict the mean difference of P100 latencies between the first and the fourth block from age, gender, frequency of migraine attacks, duration of disease, and clinical features of headache that included distribution, character, the severity of headache, photophobia, phonophobia, and nausea/vomiting. The statistical prediction of P100 latencies based on these variables is non-significant, F (11, 8) = 1.389482, P = 0.32, R2 = 0.656 [Table 3]
| Domain score | Standard coefficients | 95.0% Confidence interval for B | |||
|---|---|---|---|---|---|
| Beta | t | Sig. | Lower bound | Upper bound | |
| Age | −0.05034 | −0.81711 | 0.437521 | −0.192424582 | 0.091735349 |
| Gender | −1.00539 | −0.83776 | 0.426483 | −3.772797126 | 1.762020128 |
| Education | −0.8394 | −0.77664 | 0.459713 | −3.331737936 | 1.652937443 |
| Frequency of migraine attacks | −0.32774 | −1.44805 | 0.185634 | −0.849668501 | 0.194185093 |
| Duration of disease | −0.35923 | −0.98443 | 0.353737 | −1.200720422 | 0.482259568 |
| Distribution | 0.350881 | 0.320269 | 0.75697 | −2.175536035 | 2.87729772 |
| Character | 0.342078 | 0.375058 | 0.717365 | −1.761151708 | 2.445308399 |
| Severity | 2.371828 | 1.902379 | 0.093624 | −0.503227246 | 5.24688394 |
| Photophobia | −1.21196 | −0.43323 | 0.676295 | −7.663087067 | 5.239160923 |
| Phonophobia | 2.718994 | 1.078377 | 0.312304 | −3.095308309 | 8.533296967 |
| Nausea/Vomiting | 2.616298 | 1.952173 | 0.086701 | −0.474203387 | 5.706799043 |
Sig.: Statistical significance
The mean difference of P100 amplitude between the first and the fourth block was also used as a dependent variable for multiple regression analysis with independent variables the same as mentioned for the mean difference of P100 latencies. The statistical prediction of P100 amplitudes based on these variables is also non-significant, F (11, 8) = 0.616526, P = 0.77, R2 = 0.4587 [Table 4].
| Domain score | Standard coefficients | 95.0% confidence interval for B | |||
|---|---|---|---|---|---|
| Beta | t | Sig. | Lower bound | Upper bound | |
| Age | 0.036708 | 0.560068 | 0.590767 | −0.114433234 | 0.187849892 |
| Gender | −1.77814 | −1.39284 | 0.201154 | −4.72205046 | 1.165767213 |
| Education | −0.14299 | −0.12436 | 0.904095 | −2.79428045 | 2.508308314 |
| Frequency of migraine attacks | −0.21345 | −0.88652 | 0.401202 | −0.768660477 | 0.341768171 |
| Duration of disease | −0.21586 | −0.55608 | 0.593366 | −1.111019672 | 0.679297672 |
| Distribution | −0.20165 | −0.17303 | 0.866929 | −2.889200475 | 2.485893681 |
| Character | 0.427279 | 0.440386 | 0.671314 | −1.810091079 | 2.664649281 |
| Severity | 1.358621 | 1.02438 | 0.33563 | −1.699800426 | 4.417042227 |
| Photophobia | 0.473907 | 0.159245 | 0.877423 | −6.388657938 | 7.336471589 |
| Phonophobia | 0.02131 | 0.007945 | 0.993855 | −6.163818094 | 6.206438142 |
| Nausea/vomiting | −0.29581 | −0.20748 | 0.840815 | −3.583413316 | 2.991802029 |
Sig.: Statistical significance
There was a negative correlation between age and mean P100 amplitude difference between the first and fourth block (Pearson’s r = −0.08497, P < 0.001) [Figure 1a] and a positive correlation with headache frequency (r = 0.07438, P < 0.001) [Figure 1b]. However, there was a moderate negative correlation present between headache duration and mean P100 amplitude difference (r = −0.45682, P < 0.001) [Figure 1c]. The mean P100 latency difference between the first and fourth block correlated positively with age (r = 0.07086, P < 0.001) [Figure 1a] and negatively with headache frequency (r = −0.19043, P < 0.001) [Figure 1b] while there was a moderate positive correlation with headache duration (r = 0.33583, P < 0.001) [Figure 1c and Table 5].
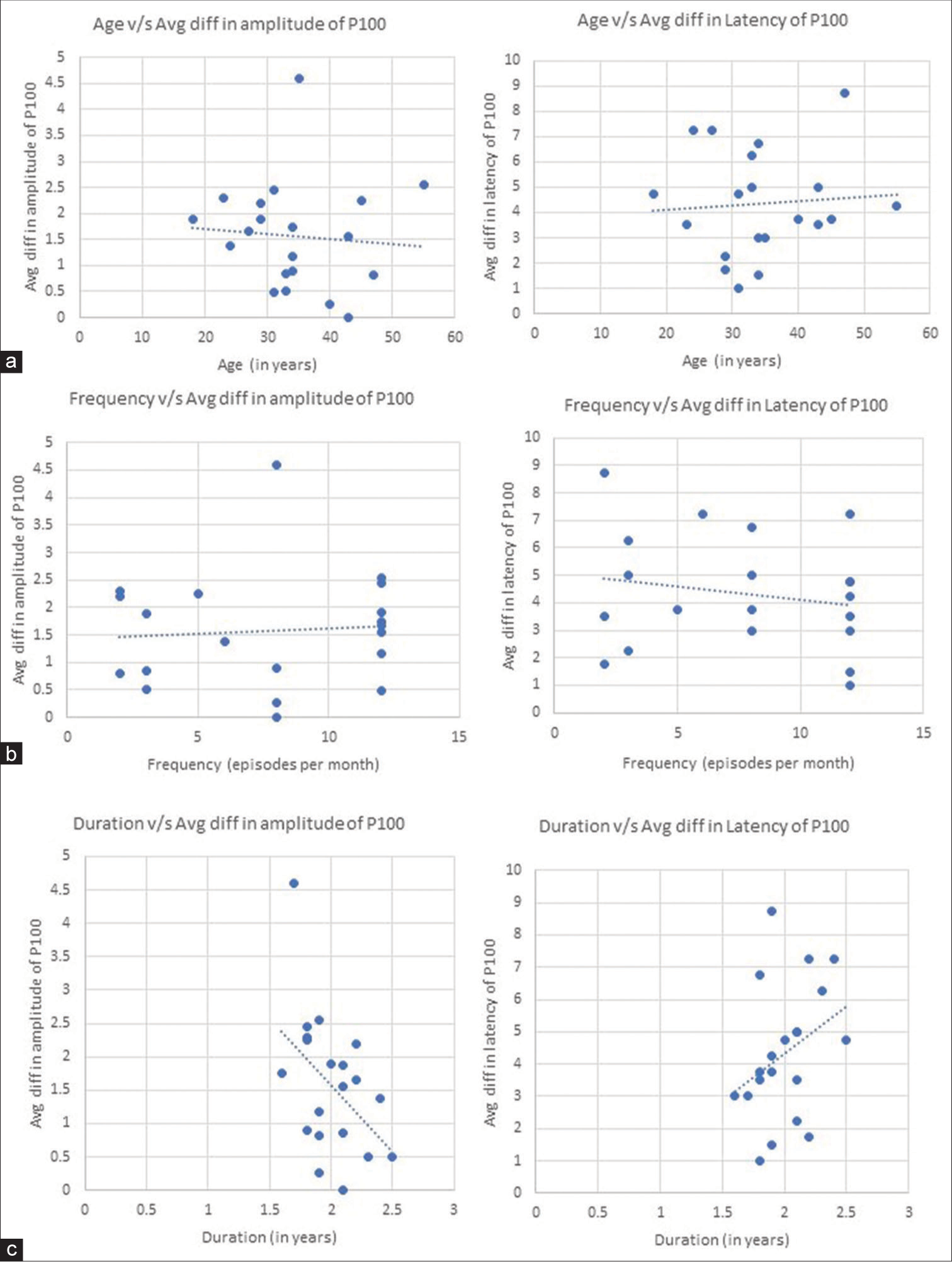
- Correlation graphs showing (a) negative correlation graph between age and mean difference (block one–block four) of P100 amplitude (Left) and positive correlation graph between age and mean latency difference (block one–block four) of P100 latency (Right). (b) Positive correlation graph between frequency of headache and mean difference (block one–block four) of P100 amplitude (Left) and negative correlation graph between frequency of headache and mean difference (block one–block four) of P100 latency (Right). (c) Negative correlation graph between duration of migraine and mean difference (block one–block four) of P100 amplitude (Left) and positive correlation graph between duration of headache and mean difference (block one–block four) of P100 latency (Right).
| Characteristics | Parameter of P100 wave | Pearson coefficient (r) | P-value |
|---|---|---|---|
| Age (in years) | Amplitude | −0.08497 | <0.001 |
| Latency | 0.070861 | <0.001 | |
| Frequency (episodes per month) | Amplitude | 0.074376 | <0.001 |
| Latency | −0.19043 | <0.001 | |
| Duration (in years) | Amplitude | −0.45682 | <0.001 |
| Latency | 0.33583 | <0.001 |
DISCUSSION
We recruited 20 controls and 20 patients (Female [12]: Male [8]) with migraine; 85% of those patients reported migraine without aura. Photophobia (45%) and nausea (55%) were the most common symptoms accompanying migraine. A majority (80%) of the patients reported throbbing pain and 65% had moderate pain. The 55% of the total patients recruited reported unilateral pain while 40% had holocranial pain. The 40% of the patients were on prophylactic medications.
In our study, subjects with migraine showed P100 amplitude habituation as compared to controls, which contradicts findings from other studies published.[1,2,7] Both controls and migraineurs exhibited habituation. This can be due to methodological differences. For instance, in the current study, which we designed to evaluate habituation, the stimulation rate and the number of averaged responses on which measures were performed were modest. In addition to this, it was found that VEP parameters vary as a function of spatial frequency.[8,9] An increase in spatial frequency results in a reduction of P100 amplitude. It has been shown that as the spatial frequency increases, visual sensitivity gradually declines. This can be attributed to the eye’s optical quality or the higher levels of visual processing (beyond the lateral geniculate nucleus) responsible for VEP production. In addition, it is suggested that sensitivity loss at high spatial frequency may result from quantal variations in light. This might be one of the reasons behind the discrepancy in the results. Furthermore, most of our patients were already on migraine prophylactic drugs and did not have migraine attacks in the recent past before the study. This result contradicts claims made in several other review articles that the lack of habituation in interictal migraineurs is a neurophysiological characteristic and the most repeatable finding.[2,7] A few trials with either negligible or no variation in the amplitude of the P100 wave were found to have similar outcomes to our study.[10-15]
When the VEP is recorded continuously for a long period (18–20 min), latency may increase while amplitude decreases. Such a prolongation of latency was noted in both migraine patients as well as in controls. However, the mean change in latencies between the migraine patients and controls was non-significant for the N75 and P100 waves, while the difference was highly significant for the N145 wave. Numerous studies that found a non-significant prolongation of P100 latency reported similar outcomes.[11-15] The VEPs can show changes in neurological function brought on by ischemic injury, aberrant neurotransmitter levels or both. The prolonged P100 latency may be brought on by the long-term repercussions of assaults, which include cerebral ischemia and edema. However, we included individuals in our study who had been suffering from migraine for only two years, which may be the cause of the non-significant lengthening of the P100 latency [Table 1]. In addition, 85% of the participants in our survey claimed to have migraine without aura. Since only 15% of migraineurs with aura were recruited in our study, a subgroup analysis was not statistically feasible. However in a study, migraine sufferers with an aura, in particular, were found to have greater P100 latency.[10]
A common occurrence in the neural system, habituation has intricated spatially and functionally dependent mechanisms. It is proposed that the cerebral cortex is regulated by excitatory interneurons that receive input from thalamocortical neurons, inhibitory intra-cortical interneurons, and subcortical connections in the brainstem involving neurotransmitters such as histamine, noradrenaline, serotonin, and dopamine. These mechanisms typically guard against cortical overstimulation.[16,17] Serotonin has extensive sensory cortex innervation, tonic pacemaker activity, and modulatory effects on cortical information processing.[18] A 1 Hz reversal rate was used in the current study, and habituation appeared to be evident in both the controls and the migraineurs. Although there is mounting evidence that migraines modify cortical excitability, it is unclear whether these alterations are the result of changed excitatory connections, damaged inhibitory networks or altered subcortical preactivation.[19] The inconsistent results in earlier VEP amplitude studies were initially thought to be due to habituation2, but this explanation now seems less likely in light of the current findings. Several investigations have indicated that migraineurs do not exhibit a lack of habituation.[6,8,20-23] In our study, we found that the duration of migraine is strongly associated with the mean P100 amplitude difference. This may imply that the severity and duration of migraines over time may be a risk factor for impaired visual cortex function. In addition to this, duration is also moderately associated with the mean P100 latency difference. We found that VEP amplitude habituation increased with migraine frequency. The average P100 latency difference was found to be decreasing with an increase in frequency. This is possibly due to the shorter time between attacks in patients with a high frequency of the condition.
There was no discernible relationship between age and the P100 wave parameter. This might be a result of the smaller sample size and dispersed age data plot.
In our study, no correlation was observed between VEP and photophobia. It may be possible that photophobia depends more on subcortical than cortical visual pathways.[22,24] These findings confirm the association between the severity of migraine clinical presentation and the behavior of neurophysiological responses.
This study has a few strengths and limitations. The primary strength of the study is its adequate sample size. Another strength is the inclusion of healthy controls for proper comparison between the two groups. The stimulation rate and low number of averaged responses on which measurements were performed in the present study, in which we aimed to evaluate habituation, are probably the primary limitations of the investigation. Second, most of our patients were already on migraine prophylactic drugs and did not have migraine attacks in the recent past before the study.
CONCLUSION
Our study results suggest that, at least in the interictal stage, lack of habituation cannot be used as a reliable neurophysiological migraine marker in different laboratories. The disparate findings in previous research are probably due to methodological discrepancies such as the stimulation rate, the number of averaged responses, and the patient recruitment strategy. It seems that migraineurs’ habituation behavior is not always hindered, but rather is affected in a subtle way that depends on stimulating stimuli. To properly understand the varying results of VEP habituation studies in migraineurs and the effects of the migraine cycle, particularly the pre-ictal phase, a long-term study is required. Drug-naïve people and those who have recently had episodes must be the subjects for future research.
Ethical approval
The research/study approved by the Institutional Review Board at Jawaharlal Institute of Postgraduate Medical Education and Research, number JIP/IEC/2021/0247, dated November 20, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia. 1992;12:221-8. discussion 186
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of visual evoked potential in migraine individuals. Int J Sci Stud. 2016;4:46-50.
- [Google Scholar]
- Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427-39.
- [CrossRef] [PubMed] [Google Scholar]
- The brain is hyperexcitable in migraine. Cephalalgia. 2007;27:1442-53.
- [CrossRef] [PubMed] [Google Scholar]
- Neuronal dysexcitability may be a biomarker of migraine: A visual evoked potential study. Clin EEG Neurosci. 2018;49:342-50.
- [CrossRef] [PubMed] [Google Scholar]
- Visual evoked potentials in migraine patients: Alterations depend on pattern spatial frequency. Brain. 1999;122:1147-55.
- [CrossRef] [PubMed] [Google Scholar]
- Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur J Neurol. 1995;2:115-22.
- [CrossRef] [PubMed] [Google Scholar]
- Visual evoked potential and spatial frequency in migraine: A longitudinal study. Acta Neurol Scand Suppl. 2009;189:33-7.
- [CrossRef] [PubMed] [Google Scholar]
- Low-contrast pattern-reversal visual evoked potential in different spatial frequencies. J Ophthalmic Vis Res. 2020;15:362-71.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormalities of visual evoked potential in migraine patients. Iran J Med Sci. 2003;28:65-8.
- [Google Scholar]
- Visual evoked potentials and brainstem auditory evoked potentials in migraine and transient ischemic attacks. Cephalalgia. 1985;5(2_suppl):53-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern-shift visual evoked responses and EEG in migraine. Headache. 1986;26:451-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern-reversal visual evoked potentials and EEG correlations in common migraine patients. Headache. 1988;28:269-71.
- [CrossRef] [PubMed] [Google Scholar]
- Multichannel visual evoked potentials in migraine. Electroencephalogr Clin Neurophysiol. 1995;96:1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of disease duration on visual evoked potentials in migraineurs. Headache. 2000;40:384-8.
- [CrossRef] [PubMed] [Google Scholar]
- Habituation and the orienting reflex: The dual-process theory revisited In: International conference on orienting reflex in humans. 1979.
- [Google Scholar]
- Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165-229.
- [CrossRef] [PubMed] [Google Scholar]
- Cortical inhibition and habituation to evoked potentials: Relevance for pathophysiology of migraine. J Headache Pain. 2009;10:77-84.
- [CrossRef] [PubMed] [Google Scholar]
- Maturation of early visual processing investigated by a pattern-reversal habituation paradigm is altered in Migraine. Cephalalgia. 2005;25:280-9.
- [CrossRef] [PubMed] [Google Scholar]
- Visual, long-latency auditory and brainstem auditory evoked potentials in migraine: Relation to pattern size, stimulus intensity, sound and light discomfort thresholds and pre-attack state. Cephalalgia. 2000;20:804-20.
- [CrossRef] [PubMed] [Google Scholar]
- Visual evoked potential latency, amplitude and habituation in migraine: A longitudinal study. Clin Neurophysiol. 2008;119:1020-7.
- [CrossRef] [PubMed] [Google Scholar]
- Long term decline of P100 amplitude in migraine with aura. J Neurol Neurosurg Psychiatry. 2000;69:507-11.
- [CrossRef] [PubMed] [Google Scholar]
- Visual evoked potential responses after photostress in migraine patients and their correlations with clinical features. J Clin Med. 2021;10:982.
- [CrossRef] [PubMed] [Google Scholar]







