Translate this page into:
Infantile subdural empyema: The role of brain sonography and percutaneous subdural tapping in a resource-challenged region
Address for correspondence: Dr. Okezie Obasi Kanu, Department of Surgery, Lagos University Teaching Hospital, PMB 12003, Surulere, Lagos, Nigeria. E-mail: daddykay143@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
This study explored the outcome of children with patent anterior fontanelles who were treated with trans-fontanelle ultrasound scan (TFUSS), which is more affordable and available than CT scan and MRI in the diagnosis of childhood intracranial pathologies and treatment of subdural empyema, in developing countries.
Patients and Methods:
Seventeen infants with post-meningitic subdural empyema, diagnosed using trans-fontanelle ultrasound alone and treated with subdural tapping over a 31-months period, were studied.
Results:
Eleven patients presented with grades II and III Bannister and William grading for level of consciousness in intracranial subdural empyema. Aspirate from 7 (41.2%) patients were sterile. The most common organisms isolated were Streptococcus faecalis 3 (17.6%), Haemophilus Influenza 2 (11.8) and Staphylococcus aureus 2 (11.8), multiple organisms were isolated in three of the patients. Ninety-four percent (94%) of the patients had good outcome. Five subjects developed hydrocephalus, one patient had a recurrence of subdural empyema, four patients had residual hemiparesis, two of the four patients had speech difficulties, while one patient (~6%) died.
Conclusion:
While CT and MRI remain the gold standard for investigating intracranial lesions, transfontanelle ultrasonography is adequate for diagnosis of infantile subdural empyema in resource-challenged areas. Percutaneous subdural tap is an affordable and effective therapy in such patients with financial challenges.
Keywords
Cranial sonography
subdural empyema
subdural taps
transfontanelle ultrasonography
Introduction
The use of brain sonography or trans-fontanelle ultrasound scan (TFUSS) in the diagnosis of childhood intracranial pathologies has been documented in the literature just like the use of subdural taps for the treatment of subdural empyema (SDE). Subdural tap was used both for diagnosis[12345] and treatment[356] of this condition but has been largely abandoned since the advent of modern imaging techniques. Computerized tomographic (CT) scans and magnetic resonant imaging (MRI) are better diagnostic tools for intracranial conditions and have become available in many centers across the world including ours. CT scan is the most common diagnostic tool used in the management of SDE and has helped to reduce mortality.[7891011] But despite the availability of these diagnostic tools, they are not affordable to all patients who visit the hospital in most parts of Sub-Saharan Africa. Children with subdural empyema (SDE), whose parents cannot afford to have an urgent CT scan, nor bear the financial burden of the surgical treatment, might deteriorate leading to fatal outcome. Brain sonography being far cheaper than CT or MRI is therefore useful in such patients with patent anterior fontanelle.
Patient selection
Patients who fall into the above category who presented with features of postmeningitic intracranial suppuration, in whom diagnosis of SDE were made by TFUSS and treated using only Subdural taps were included in this study. The study period was from February 2006 to August 2008.
All patients who had subdural empyema and whose parents were poor and could not afford the cost of CT and whose diagnosis were made using brain sonography (TFUSS) and subsequently treated by subdural tap were included in this study. These patients had been diagnosed and treated for meningitis or currently on treatment when they were referred to the Neurosurgical team. The Otorhinolaryngologist assessed the patients for ear, nose and throat (ENT)-related causes of intracranial suppuration.
Patients who had CT bran scan were excluded.
Controls
This is a prospective observational study. We considered that a control with patients who had CT scan and TFUSS was not necessary to access accuracy of diagnosis since the patients would be treated by subdural tap, which confirmed the diagnosis.
Patients and Methods
The biodata of the patients were collected. The presenting clinical features, the location of the empyema, total volume of aspirate, bacteriology of samples, treatment outcome and residual deficits were documented. Diagnosis was made by cranial sonography alone.
All patients were treated using a sterile 18-guage plastic cannula with metal trocar. All taps were performed by aseptic technique. The use of plastic cannulae made it possible to remove almost all subdural collection in one procedure using a single tap with multiple syringes while the cannula remained in place. Patients with bilateral subdural empyema had the procedure repeated on the contralateral side at the same sitting.
A repeat TFUSS was done within 24 h to re-evaluate and confirm complete removal of the subdural collection.
Intravenous antibiotics were continued for 2 to 4 weeks post-drainage depending on clinical status. The therapy was continued and completed with oral antibiotics subsequently.
The duration of follow-up was 1 to 3 years.
Results
Age and gender
There were 17 patients in this study: 10 males, 7 females. Age range was from 2 months to 14 months with a mean age of 8 months.
Etiology
All the patients in this study were cases of post-meningitic intracranial suppuration. Five patients had bilateral subdural collection while the rest where unilateral. The average volume of empyema aspirated was 34.3 ml (20-48 ml).
Clinical presentation
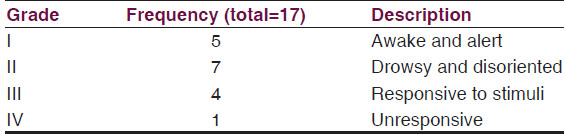
The most common clinical presentation was bulging anterior fontanelle with increased occipitofrontal circumference (OFC) as observed in all patients. Other clinical presentations were fever, meningeal irritation, altered consciousness focal deficits and seizures as shown in Table 1.

Bannister and William[10] grading of level of consciousness was Grade I and II in 12 patients while 5 patients presented below grade III and IV [Table 2].
Therapy
A total of 18 procedures were performed in the 17 patients. One patient had a recurrence of subdural empyema which was re-drained. All the patients received a combination of intravenous antibiotics to ensure broad spectrum cover. All the patients had received antibiotics for treatment of meningitis before referral for neurosurgical intervention. Antibiotics were changed or continued based on sensitivity pattern and/or clinical improvement especially when the aspirate was sterile. Four patients additionally received intraventricular Vancomycin prescribed by the pediatric neurologist. All the seven patients who presented with seizures received anti-convulsant therapies and were seizure free at 1 year.
Five of the patients developed hydrocephalus of post-infective type and subsequently treated with endoscopic third ventriculostomy (ETV), three of whom failed and had ventriculo-peritoneal shunt insertion with good outcome. There was residual hemiparesis in four patients which necessitated physiotherapy and all resolved at 6 months post therapy. Two of the four patients had speech difficulties and were managed by the speech therapist.
Bacteriology
Aspirates were subjected to microscopy, culture and sensitivity (MCS) studies. The organisms isolated and their frequencies are shown in Table 3. The aspirate was sterile in seven patients (41.2%). The most common organism isolated was Streptococcus faecalis (17.6%). Haemophilus Influenza and Staphylococcus aureus ranked second. There were multiple organisms isolated in three of the patients. All the aspirates had glucose level less than 20 mg% and white blood cell (WBC) differentials with polymorphs ≥75%.

Outcome [Table 4]
Using the Henk W. Mauser[12] grading system for morbidity of survivors, 94% of the patients (Grade A and B) had good outcome with only minor disabilities where present [Table 4].
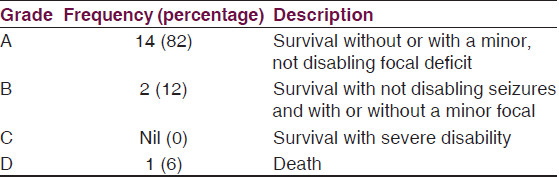
Speech has improved in the two patients with residual speech deficit undergoing speech therapy. Only one patient (~6%) died.
Discussion
The purpose of this study is to demonstrate the usefulness of both sonography and subdural taps in the early diagnosis and treatment of subdural empyema especially in resource-challenged regions where the medical insurance is ineffective yet and diagnostic delays could increase the morbidity and mortality of SDE in such conditions. It has been shown in other series in the literature that outcome is favorable when diagnosis is made on time followed by early intervention (surgical evacuation and antibiotics therapy). Mortality often results from delay in referral[13] or delay in diagnosis and commencement of therapy.[13141516] The cost of brain CT scan is about $350 - $400USD equivalent while transfontanelle sonography costs about $20USD. Though CT and MRI have been proven to be more effective in diagnosis, patients still have to bear the cost of these investigations. In most developing countries the Gross Domestic Product (GDP) is low; health insurance schemes are nonexistent or at best ineffective. In a situation where patients and relations are poor and hardly have enough for the diagnosis and subsequent surgical therapy, there will be a delay in making diagnosis and patients might deteriorate, leading to increased morbidity or even fatal outcome. A patent fontanelle and a cheap TFUSS that confirms diagnosis would have reduced the time of diagnosis. The added advantage of using sonography is that it could be repeated severally without the risk of radiation associated with the use of CT brain scan. Figure 1 is a typical sonography of one of the patients with bilateral subdural collection.
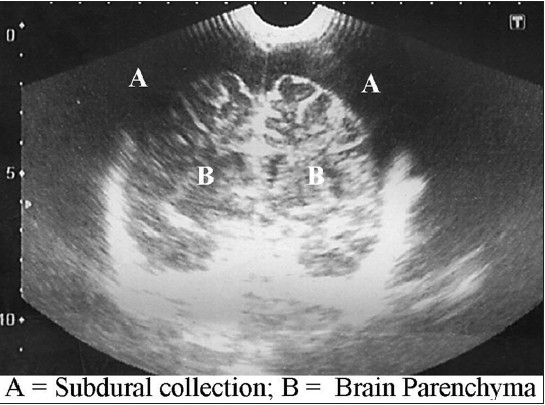
- Transfontanellesonography of a child with bilateral subdural collection (annotated)
In our study, it was possible to accurately diagnose the presence of subdural empyema in all patients. The history of previous or on-going meningitis with the clinical features enumerated helped to clinch the diagnosis.
The use of percutaneous subdural tap as described above made it possible to evacuate the subdural collection and this was carried out effectively. Burr holes and craniotomies have been used in older patients and though some reports favor craniotomy;[9171819] there is still no consensus on the method of surgical evacuation and this varies with age and the stage of the abscess.[20] Percutaneous aspirations through an open fontanelle and twist drill have been used as minimally invasive techniques with satisfactory results.[45212223] Most authors favor early drainage and antibiotics therapy to reduce morbidity, clinical deterioration and mortality.[91819] Antibiotics therapy alone was not adequate for treatment when neurologic deficits have already set in.[182425]
The aspirates were sterile in 7 patients (41.2%) and the commonest organism isolated was Streptococcus faecalis (17.6%). Curless[3] recorded negative culture stains in some of his patients who had H. Influenza meningitis. Eighteen of 37 cultures performed by Mauser and Tulleken[12] were sterile. These results may be due to the fact that antibiotics previously administered for the treatment of meningitis interfered with the culture yield, but the low glucose level and high polymorph WBC differentials in these patients suggest ongoing bacterial activity.
Only one patient had a recurrence which was treated with a repeat tap. This patient was seen at Bannister and Williams’[10] grade IV level of consciousness at the time of transfer from a private facility. The parents did not accept transfer to a tertiary hospital till the level of consciousness dropped significantly. She had a prolonged course and later died. This is the only mortality recorded in our series (~6%). Mortality in the literature is closely related to delay in referral or delay in treatment.[781011]
Outcome in our study compares favorably with Jacobson and Farmer[5] though most of their six patients were managed with burr holes. Curless[3] on the other hand reported that repeated subdural taps produced satisfactory results in eight of nine patients in his series. Diagnoses in these patients were done using CT scan in some and subdural taps in others. The patient populations being small would not allow objective comparison of the effect of imaging modalities on overall outcome but certainly CT scan is superior to TFUSS used in this study. However, Farmer and Wise[26] lost four of their eight patients in their series and this may be attributable to delayed diagnosis due to lack of CT scan. There is no record of TFUSS being used in any of their patients.
Most residual deficits resolved satisfactorily at 6 months to one year. Patients with hydrocephalus had endoscopic third ventriculostomy (ETV) or ventriculoperitoneal shunt (VPS) when the ETV failed or cisterns were significantly scarred. Anticonvulsant therapy was however continued for a period of 2 years in those who had seizures though they were seizure free at 1 year. Two out of 9 patients reviewed by Curless[3] had recurrence of subdural empyema. One of his patients had severe psychomotor retardation and another patient had sustained speech problems 2 years after infection. One patient was lost to follow-up. There was no record of hydrocephalus in his series. We believe however that the presence of hydrocephalus is an indication of the severity of the infective process and not a complication of treatment.
It is not possible to compare the present result with the group managed with CT scan and burr holes because the local data presently consists of adult patients in whom subdural empyema were managed with burr holes and craniotomy, and the etiology is varied.
Conclusion
While CT and MRI remain the gold standard for investigating intracranial lesions, and will be deployed where available, trans-fontanelle ultrasonography is adequate in making diagnosis of infantile subdural empyema especially in resource-challenged areas. Subdural empyema in children with patent anterior fontanelle can be satisfactorily treated with percutaneous subdural taps with low recurrence rates and low mortality rates. This saves cost and surgical time. Majority of culture results are organism-negative and broad spectrum antibiotics are effective in providing satisfactory antibacterial cover. The effectiveness of intrathecal antibiotics and mode of delivery for SDE requires consideration and evaluation in subsequent studies.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Bacterial meningitis: Newer concepts of pathophysiology and neurologic sequelae. Pediatr Clin North Am. 1976;23:541-56.
- [Google Scholar]
- Subdural empyema in infant meningitis: Diagnosis, therapy, and prognosis. Childs Nerv Syst. 1985;1:211-4.
- [Google Scholar]
- Osteomyelitis and epi-, extra-, and subdural abscesses. Clin Neurosurg. 1966;14:239-55.
- [Google Scholar]
- Subdural empyema complicating meningitis in infants: Improved prognosis. Neurology. 1981;31:190-3.
- [Google Scholar]
- Demonstration of purulent bacterial intracranial infections by computed tomography. AJR Am J Roentgenol. 1976;127:155-65.
- [Google Scholar]
- Factors affecting the outcome in subdural empyema. J NeurolNeurosurg Psychiatry. 1987;50:1136-41.
- [Google Scholar]
- Subdural empyema: A review of 29 cases. J Neurol Neurosurg Psychiatry. 1964;27:422-34.
- [Google Scholar]
- Controversies in the management of subdural empyema. A study of 41 cases with review of literature. Acta Neurochir (Wien). 1990;102:25-32.
- [Google Scholar]
- Salmonella intracerebral and subdural abscess-report of two cases. Postgrad Med J. 1987;63:373-5.
- [Google Scholar]
- Penetration of brain abscess by systemically administered antibiotics. J Neurosurg. 1973;38:705-9.
- [Google Scholar]
- Subdural empyema: A retrospective study of 15 patients. Can J Surg. 1984;27:283-5, 288.
- [Google Scholar]






