Translate this page into:
Identifying autonomic nervous system dysfunction in acute cerebrovascular attack by assessments of heart rate variability and catecholamine levels
Address for correspondence: Dr. Eşref Akıl, Department of Neurology, Dicle University, Diyarbakir - 21280, Turkey. E-mail: esrefakil@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
This study aimed to evaluate changes in the autonomic nervous system caused by cerebral lesions due to acute stroke. We assessed heart rate variability and catecholamine levels in lieu of stroke lesion localization.
Materials and Methods:
A total of 60 stroke patients and 31 healthy controls were enrolled in the study. Plasma epinephrine and norepinephrine levels were measured on the first, third, and seventh days following the stroke event. Heart rate variability was evaluated with time-domain and frequency-domain analyses via 24-hour Holter monitor recordings.
Results:
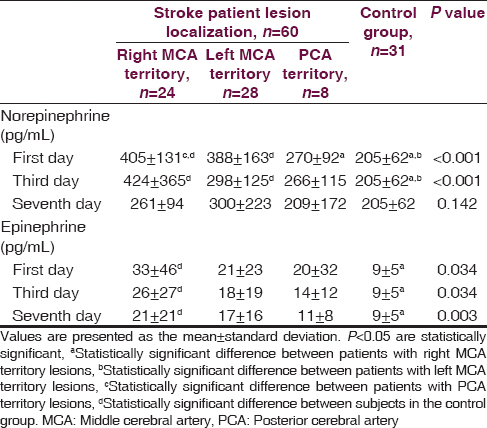
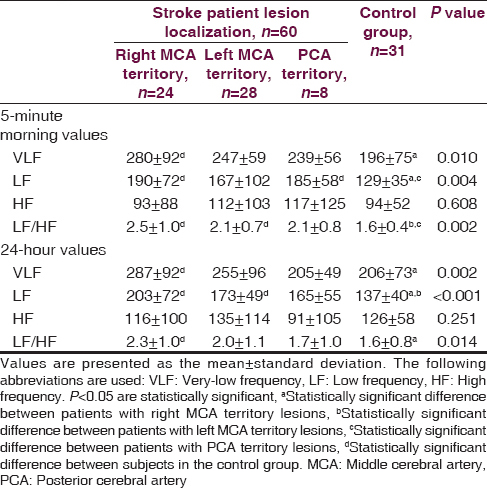
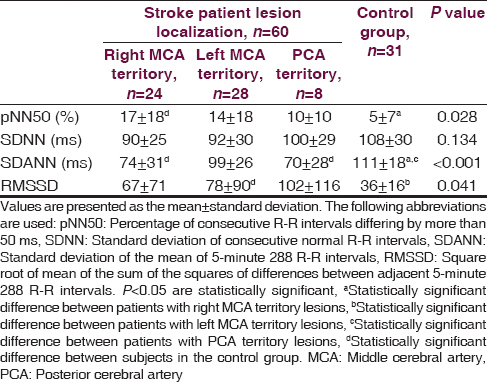
On the first and third day following the stroke, norepinephrine levels were significantly higher in all patient groups as compared to controls. Epinephrine levels on the first, third and seventh days after the stroke were significantly higher in patients with lesions in the right middle cerebral artery territory than controls. In frequency-domain analysis, patients with right middle cerebral artery territory lesions had greater low frequency and low frequency to high frequency ratio values than controls. Time-domain analysis revealed significant decreases in the standard deviation from the mean for 5-minute 288 R-R intervals in patients with lesions in the right middle cerebral artery and posterior cerebral artery territory when contrasted with controls. Patients with lesions in the right middle cerebral artery territory demonstrated the highest increase in the percentage of consecutive R-R intervals differing by more than 50 ms (pNN50) as compared to the control group.
Conclusion:
These findings indicate that autonomic dysfunction favoring an increase in sympathetic activity occurs in acute stroke patients.
Keywords
Autonomic nervous system
catecholamines
heart rate variability
stroke
Introduction
Despite major progress in the treatment of acute stroke it remains one of the most devastating neurological conditions and substantial economic costs are incurred due to stroke-related disability.[1] The brain damage sustained by stroke may lead to death or neurological deficits. Cardiac complications such as arrhythmias, ischemic heart injury, or sudden death resulting from cardiovascular autonomic dysfunction is frequently observed following acute stroke.[2] Heart rate is influenced by both the sympathetic and parasympathetic autonomic systems.[3] Thus, analysis of heart rate variations provides a means to investigate autonomic nervous system (ANS) function. Directly measuring the peripheral activity of the ANS in humans is difficult. Hence, noninvasive, rapid and repeatable tests such as measuring neuro-immune biomarker levels, sympathetic skin response via electrophysiological techniques, and heart rate variability (HRV) are utilized. The reliability of such tests that evaluate autonomic disorders has been optimized and standardized in previous studies.[45] HRV analysis is a noninvasive method utilized to evaluate the autonomic regulation of the cardiovascular system, which reflects both sympathetic and parasympathetic influences.[6] HRV measurement is performed with a 5-minute short-term or a 24-hour long-term electrocardiogram (ECG) recording using time-domain, frequency-domain, and geometric and nonlinear methods. Time-domain HRV analysis is preferred for long-term recordings and provides adequate information regarding cardiac autonomic function. Frequency-domain analysis is preferred for short-term measurements since it is more appropriate for certain medical conditions and is more easily interpreted. It has been demonstrated that time-domain parameters and frequency-domain parameters are strongly correlated with each other.[7] Catecholamines such as dopamine, norepinephrine and epinephrine all play key roles in the regulation of many physiological processes and have various effects on neurological, psychiatric, endocrine and cardiovascular conditions.[8] Plasma norepinephrine concentration is considered as an indicator of peripheral sympathetic activity. Norepinephrine is a neurotransmitter found in both the brainstem and the peripheral sympathetic nervous system. There is a close association between the release of norepinephrine into the synaptic space and an increase in sympathetic tone. In our study we evaluated the ANS changes experienced by acute stroke patients according to the localization of the stroke lesion. HRV time-domain and frequency-domain parameters and plasma catecholamine levels were analyzed so to demonstrate the relationship between sympathetic and parasympathetic function and stroke lesion location.
Materials and Methods
A total of 60 patients that presented with stroke and were admitted in the Neurology Clinic of Dicle University Faculty of Medicine within a year were enrolled in the study. A group of 31 healthy volunteers comprised the control group. Exclusion criteria from the study were patients with diabetes, a history of arrhythmia and/or stroke, cardiac valve prosthesis, advanced heart failure, and renal and/or hepatic insufficiency. In addition, patients taking medications such as digoxin, lithium, tricyclic antidepressants, theophylline, levodopa, beta-blockers, and calcium channel blockers, which are known to influence ECG readings and cardiac rhythm on Holter monitoring, were also excluded. Patients that presented to the hospital more than 24 hours following the stroke, patients that had no identifiable cerebral lesion on magnetic resonance imaging (MRI), and patients with cerebral lesions < 15 mm on MRI were eliminated from the study. The study protocol was approved by the Dicle University Ethics Committee, and informed consent was obtained from every participant.
All patients underwent detailed general and neurological examinations. They were questioned about stroke risk factors including hypertension, diabetes, coronary artery disease, and heart failure. On admission, a complete blood count, routine blood biochemistries, hepatic and renal function tests, and blood electrolyte measurements were obtained. Epinephrine and norepinephrine levels were measured on the first, third and seventh day following the stroke event. Catecholamine levels were measured from plasma of venous samples obtained between 8:00 a.m. and 9:00 a.m. via high performance liquid chromatography (HPLC) (Shimadzu LC 10A). Serial 12-lead ECG recordings with long DII derivations were obtained in addition to 24-hour Holter monitoring. All patients received brain imaging via MRI.
Recordings were performed with 24-hour Holter monitoring and analyzed with Delmar-Impresario system and software (Delmar-Impresario Medical Systems, Irvine, California, USA). The analyzed data and standard measurement criteria were evaluated according to European Society of Cardiology guidelines.[9] HRV time-domain and frequency-domain analyses were performed to detect sympathetic and parasympathetic ANS influences on the cardiovascular system. HRV time-domain parameters include mean normal-to-normal (NN) intervals, standard deviation of all NN intervals (SDNN), standard deviation of the averages of NN intervals in all 5-minute segments of the entire ECG recording (SDANN), the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD), number of pairs of adjacent NN intervals differing by more than 50 ms in the entire ECG recording (NN50), and NN50 count divided by the total number of all NN intervals (pNN50). Indexes (RMSSD, pNN50) calculated based on the differences between NN intervals are short-term measurements and reflect high-frequency variations in heart rate. These are completely independent of diurnal or any other effects on heart rate and reflect variations in the ANS regulated via the vagal route.[10] Diurnal interaction is in question for the variables directly calculated from NN interval (SDNN, SDANN, SDNN index) and contribution of short-term changes in heart rate that occur due to respiration is little.[7] HRV frequency measurements include high frequency (HF), low frequency (LF), medium frequency (MF), very low frequency (VLF), and ultra-low frequency (ULF). Among these frequency bands mostly LF, HF and the LF to HF ratio (LF/HF) are used. An increase in HF indicates a parasympathetic effect, whereas an increase in LF indicates a sympathetic effect.[9] It has been demonstrated that time-domain parameters and frequency-domain parameters are strongly correlated with each other; LF, ranging from 0.04 to 0.15 Hz, was utilized to determine sympathetic and parasympathetic nervous system influences on HRV. HF, ranging from 0.15 to 0.4 Hz, only measured parasympathetic nervous system influences on HRV. VLF, ranging from 0.003 to 0.04 Hz, was also assessed. The LF/HF ratio, which is an indirect index of the sympathetic nervous system impact on HRV, was calculated as well. For frequency analysis, 24-hour as well as 5-minute recordings were taken starting at 8:00 a.m.
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 11.5. Descriptive statistics were expressed as frequency tables and cross tables for categorical variables and as the mean and standard deviation for numerical variables. Chi-squared test or Fisher's exact test was used for analyzing categorical variables. Relationships between the variables were examined by calculating Pearson's and Spearman's correlation coefficient. Multiple group analyses were performed using the one-way analysis of variance (ANOVA) test. A P value less than 0.05 was considered statistically significant.
Results
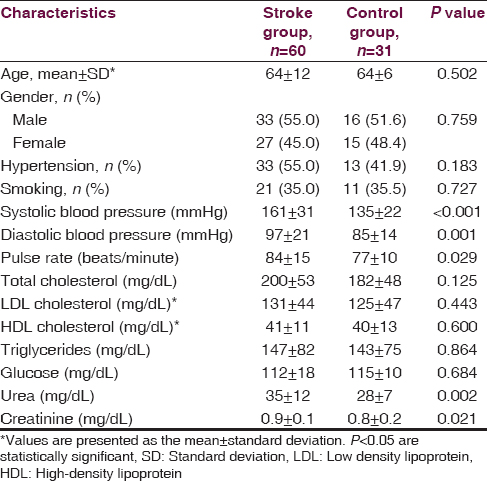
A total of 39 out of the 60 stoke patients were diagnosed with ischemic stroke, whereas 21 patients were diagnosed with hemorrhagic stroke. A lesion in the right middle cerebral artery (MCA) territory was identified in 24 patients and a lesion localized in the left MCA territory was observed in 28 patients. Only eight patients had lesions in posterior cerebral artery (PCA) territory. The stroke patients and the healthy control group were similar with regard to age, gender, hypertension and smoking history, glucose level, and lipid profile. Mean blood pressure, pulse rate, urea, and creatinine levels were higher in stroke patients as compared to the control group (P < 0.005) [Table 1]. Norepinephrine levels measured on the first and third days following the stroke event were significantly higher in patients with right and left MCA territory lesions when contrasted with controls. Norepinephrine levels in patients with right MCA territory lesions were significantly greater than patients with PCA territory lesions (P < 0.001). No differences were found among all groups in terms of norepinephrine levels measured on the seventh day (P = 0.142). Epinephrine levels measured on the first, third and seventh days were significantly higher in stroke patients with right MCA territory lesions than in controls (P = 0.034, P = 0.034, P = 0.034) [Table 2]. Among stroke patients, the most prominent HRVs were observed in patients with right MCA territory lesions. Five-minute recordings at 8:00 a.m. and 24-hour recordings on the first day following the stroke event demonstrated that VLF (P = 0.01), LF (P = 0.004) and LF/HF (P = 0.002) values were significantly higher in patients with right MCA territory lesions as compared to controls (P < 0.005). Although VLF, LF and LF/HF values were higher in patients with right MCA territory lesions, the difference was not significant when compared to patients with left MCA territory and PCA territory lesions. When comparing patients with the three different stroke localizations, it was determined that HF was most suppressed in patients with PCA territory lesions. However, no significant difference was detected between stroke patients and the control group in terms of HF values (P > 0.005) [Table 3]. Comparisons for time-domain parameters between stoke patient and control groups revealed that SDNN values were lower in stroke patients than in the controls; however, there were no significant differences in SDNN values between the different stroke localizations (P = 0.134). A significant decrease was observed in SDANN values in patients with right MCA territory and PCA territory lesions when compared with the control group (P < 0.001). RMSSD values were higher in all stroke patients than in controls, but the difference was significant only for patients with left MCA territory lesions (P = 0.041). Among all stroke patient groups, the highest increase in pNN50 was observed in patients with right MCA territory lesions. This increase in pNN50 was significant when contrasted with the control group (P = 0.028) [Table 4]. There were no differences between the ischemic stroke and the hemorrhagic stroke groups in terms of VLF, LF and LF/HF values, serum level of norepinephrine and epinephrine. In all study participants, correlation analysis revealed a moderate correlation between LF/HF and the serum level of norepinephrine on the first days (r = 0.281, P = 0.007), as well as with the serum level of norepinephrine on third day (r = 0.289, P = 0.006). No correlation was found between LF/HF and epinephrine, as well as with the serum level of norepinephrine on seventh day (P > 0.005).
Discussion
In our study, we did not identify significant differences between acute stroke patients and controls in terms of SDNN and RMSSD, which are among the time-domain recordings of HRV that indicate changes in parasympathetic activity. No differences were observed between stroke patients and controls in terms of HF values, which were among the frequency-domain short- and long-term recordings. However, decreases in SDANN and increases in pNN50, which are HRV time-domain recordings indicating sympathetic changes, were observed in acute stroke patients when contrasted with controls. These findings along with an increase in LF demonstrated that acute stroke patients had greater levels of sympathetic activity, especially in patients with right MCA territory lesions. In acute stroke patients, an increase in the LF/HF ratio, which is an indicator of sympathetic-parasympathetic tone balance, along with increases in plasma norepinephrine and epinephrine levels were observed. These findings were most prominent in stroke patients with right MCA territory lesions and revealed that autonomic dysfunction favored sympathetic activity. Acute stroke causes myocardial injury, electrocardiographic abnormalities, cardiac arrhythmias, and ultimately death if central autonomic control is severely impaired.[11] HRV assessment is the primary method for detecting cardiac autonomic dysfunction and for predicting prognosis in acute stroke patients.[1012] Korpelainen et al.[13] reported reduced HRV due to the impact of left-sided lesions localized in the insula in patients with acute left cerebral hemisphere strokes. Colivicchi et al.[14] reported a decreased HRV and arrhythmias in stroke patients with right-sided involvement of the insula and suggested that the presence of right-sided insula involvement exerted an effect on increased early mortality. These studies demonstrated that sympathetic cardiovascular tone increases when the right insular cortex is stimulated, and parasympathetic activation occurs when the left insular cortex is stimulated.[1516] Another study demonstrated an increase in arrhythmias such as premature ventricular contractions, supraventricular contractions, or supraventricular tachycardias together with a reduced SDNN which were observed in patients with a cerebral hemispheric stroke. However, such arrhythmias and decreased SDNN were more prominent in stroke patients with lesions in the right insular cortex.[17] In a study by Pandian et al.,[18] it was reported that sympathetic changes were predominant in a cohort of mild-to-moderate stroke patients as compared to healthy controls. Several other studies suggested that autonomic cardiovascular failure was observed in stroke cases resulting from parasympathetic dysfunction.[19202122] In our study, cerebral stroke lesion localization was specified, but insular cortex involvement was not investigated. As such, we could not determine whether an abnormal HRV was attributed to a lesion in the insular cortex. We found that SDANN values, an indicator of sympathetic activity, decreased in 24-hour HRV time-domain analysis in patients with right MCA territory lesions. SDNN values, which are indicators of parasympathetic activity on the sinus node, demonstrated no significant differences between all groups.
A close association has been reported between plasma norepinephrine levels in acute stroke patients and increased peripheral sympathetic activity. Specifically, increases in norepinephrine levels suggest a pathological over-activation of the ANS.[23] Moreover, associations between increases in serum troponin I and plasma epinephrine concentrations after acute ischemic stroke have been identified. This correlation suggests that increases in troponin I levels indicate sympathetic activation. Moreover, further evidence was found demonstrating that myocardial injury in acute stroke patients results from sympathetic nervous system activation.[24] In our study, troponin I and creatine kinase (CK)-MB levels were not measured, but we assessed plasma norepinephrine and epinephrine levels that directly reflect ANS function. We found that catecholamine levels tend to be higher in acute stroke patients, which suggests that these patients have greater levels of sympathetic activity. In a study by Strittmatter et al.,[25] cerebral hemisphere stroke lesions caused greater increases in norepinephrine level increments as compared to stroke patients with brainstem and cerebellum lesions. On the other hand, stroke patients with brainstem lesions had lower epinephrine levels than patients with right cerebral hemisphere lesions. Myers et al.[26] reported higher norepinephrine in patients with brainstem infarctions than in patients with cerebral hemisphere infarctions. In a study by Orlandi et al.,[27] they found that the imbalance between adrenergic and cholinergic systems favored an increase in sympathetic activity on the first day following the stroke event. Moreover, they reported that these changes substantially decreased on the third day after the stroke and over time the symptoms resolved. In our study, plasma norepinephrine levels were increased during the first and third days following the stroke event for all stroke patients as compared to healthy controls. Yet this increase in norepinephrine levels was greatest in stroke patients with right MCA territory lesions. On the seventh day, however, norepinephrine levels were slightly higher in stroke patients as compared to controls without statistical significance. We observed that plasma catecholamine levels were very high during the acute stroke period and then decreased over time. These results suggest that autonomic nervous dysfunction developed within the first days following the stroke. Cardio-autonomic function is directly increased by an increase in sympathetic activity or a decrease in parasympathetic activity. It should be emphasized that sympathetic nervous system disinhibition due to acute stroke may lead to the development of cardiac complications.
Conclusion
In conclusion, among acute stroke patients, significant autonomic dysfunction was determined in patients with right MCA territory lesions. Decreases in SDANN and increases in pNN50 in HRV time-domain analysis along with increases in LF and VLF in HRV frequency-domain analysis all suggest cardiovascular autonomic dysfunction. Plasma norepinephrine and epinephrine levels, which are among the measurable biochemical parameters of autonomic function, were high during the early phases after the stroke event. Also, high levels of these catecholamines were most pronounced in patients with right MCA territory lesions. Together these findings suggest autonomic dysfunction in the form of increased sympathetic activity. In order to prevent complications such as arrhythmias and sudden death in stroke patients, close vitals monitoring and prompt treatment is necessary, particularly during the acute phase after the stroke. Rigorous heart monitoring and rapid treatment responses may favorably affect clinical prognosis. To better study ANS imbalances in the setting of acute stroke, we recommend that larger scale and long-term prospective studies are performed to further assess these findings.
Acknowledgement
We are grateful to Dicle University DUBAP for their sponsorship about English editing of this manuscript.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(Suppl):S85-90.
- [Google Scholar]
- Cerebrogenic cardiac arrhythmias: Cortical lateralization and clinical significance. Clin Auton Res. 2006;16:6-11.
- [Google Scholar]
- Autonomic diseases: Clinical features and laboratory evaluation. J Neurol Neurosurg Psychiatry. 2003;74(Suppl 3):iii31-41.
- [Google Scholar]
- Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46:873-80.
- [Google Scholar]
- Sympathetic skin responses in hemiplegic patients with and without complex regional pain syndrome. Neurol India. 2006;54:279-82.
- [Google Scholar]
- Heart rate variability parameters correlate with functional independence measures in ischemic stroke patients. J Electrocardiol. 2002;35(Suppl):243-6.
- [Google Scholar]
- Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88-101.
- [Google Scholar]
- Clinical catecholamine neurochemistry: A legacy of Julius Axelrod. Cell Mol Neurobiol. 2006;26:695-702.
- [Google Scholar]
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043-65.
- [Google Scholar]
- Reappraisal of heart rate variability in acute ischemic stroke. Kaohsiung J Med Sci. 2011;27:215-21.
- [Google Scholar]
- Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012;11:179-88.
- [Google Scholar]
- Autonomic dysfunction and risk stratification assessed from heart rate pattern. Open Neurol J. 2010;4:39-49.
- [Google Scholar]
- Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke. 2005;36:1710-5.
- [Google Scholar]
- The anatomy and physiology of cortical mechanisms of cardiac control. Stroke. 1993;24(Suppl):I3-5.
- [Google Scholar]
- Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131-40.
- [Google Scholar]
- Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35:2094-8.
- [Google Scholar]
- Autonomic nervous activity during sleep in middle cerebral artery infarction. Cerebrovasc Dis. 1998;8:118-23.
- [Google Scholar]
- Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction. Stroke. 1996;27:2059-63.
- [Google Scholar]
- Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke. 1999;30:1307-11.
- [Google Scholar]
- Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57:833-8.
- [Google Scholar]
- Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc Dis. 2007;23:260-6.
- [Google Scholar]
- Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol. 2003;50:30-8.
- [Google Scholar]
- Transient autonomic nervous system dysfunction during hyperacute stroke. Acta Neurol Scand. 2000;102:317-21.
- [Google Scholar]






