Translate this page into:
Hyperdense large artery sign in meningitis: A marker of ominous thrombogenic potential of pneumococcus?
Address for correspondence: Dr. Deb Kumar Mojumder, Department of Neurology, School of Medicine, Texas Tech University Health Sciences Center, 3601 4th Street, Lubbock, TX - 79430, United States. E-mail: deb.mojumder@ttuhsc.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hyperdensity in the middle cerebral artery (MCA) or posterior cerebral artery (PCA) on non-contrast head CT, suggests the presence of a thrombus inside these vessels, often referred to as the “MCA sign” or “PCA sign” respectively. These two signs are classically associated with strokes secondary to cardiovascular etiologies and are only infrequently reported with other types of stroke. Whereas stroke is a recognized complication of pneumococcal meningitis hyperdense large vessel sign (in this case a combination of MCA and PCA) has not been previously reported. We report a case of rapidly progressive pneumococcal meningitis that presented as acute stroke involving large vessels in the vicinity of the circle of Willis in a patient with a history of non-Hodgkin lymphoma (NHL) in remission for 6 years. This patient had received a week of high dose steroids before admission. Head CT scan on admission showed the presence of hyperdense MCA and PCA signs. The patient rapidly deteriorated and a follow-up head CT revealed diffuse brain edema and increased density in the basal cisterns without evidence of sub arachnoid hemorrhage. Tc99m exametazime brain flow scan showed no intracerebral blood flow both supra and infratentorially. Steptococcus pneumoniae, NHL cells and high-dose steroid use can upregulate tissue factor synthesis and may have led to a hypercoagulable state via activation of the extrinsic pathway in the large intracerbral arteries.
Keywords
Extrinsic pathway of coagulation
hyperdense large artery sign
hyperdense middle cerebral artery sign
hyperdense posterior cerebral artery sign
pneumococcal meningitis
streptococcus pneumoniae
Introduction
Hyperdense middle cerebral artery (MCA) or posterior cerebral artery (PCA) sign, which are referred to cumulatively as “hyperdense large artery sign,” has been described as increased attenuation in the course of the proximal MCA or proximal PCA. Hyperdense large artery sign is mostly associated with large MCA or PCA territory infarction seen in noncontrast-enhanced computed tomography (NCCT).[12] Evidence indicates a thrombus or an embolus inside either the MCA or the PCA as the cause for the attenuation of the computed tomography (CT) scan signals with the hyperdense cerebral artery sign.[3] Other conditions causing hyperdense cerebral artery signs include: High hematocrit, calcification in the vessel walls associated with diabetes or hypertension,[4] HSV encephalitis,[5] posttraumatic edema and reversible posterior leukoencephalopathy.[1]
Although Streptococcus pneumoniae, currently the most common bacterial meningitis worldwide (for review see[6]), is known to cause localized ischemia in either the peripheral vascular system[78] or the cerebrovascular system,[910] to the best of our knowledge its association with dense cerebral large artery sign has not been reported. This case highlights the catastrophic consequences of the co-existence of conditions that can potentially activate the extrinsic pathways in patients with Pneumococcus meningitis.
Case Report
A 46-year-old right-handed white male was admitted with altered mental status. He presented with a 24-h history of severe headache and vomiting. The patient reported exacerbation of previous back pain for the preceding week that was treated with daily oral cyclobenzaprine, acetaminophen and hydrocodone, methocarbamol and prednisone 80 mg. The patient woke up feeling feverish. Later in the day, he experienced increasing headache, vomiting and worsening lower back pain. He was admitted to the hospital that evening because of lethargy and mental confusion. On admission, he was lethargic but was able to follow commands. He reported severe headache but denied photophobia, phonophobia or neck pain. His medical history was significant for follicular non-Hodgkin's lymphoma, for which he had received total body irradiation followed by allogenic sibling T-cell infusion (sister, identical HLA status) 6 years earlier. His last visit to the oncologist was 4 months ago, where he was found to have no evidence of disease (Karnofsky Performance Scale Index = 100).[11] Specifically, this patient did not have lymph node enlargement, subcutaneous nodule or clinical features of graft versus host disease. The patient worked as a full-time football coach. He seldom drank alcohol and never smoked.
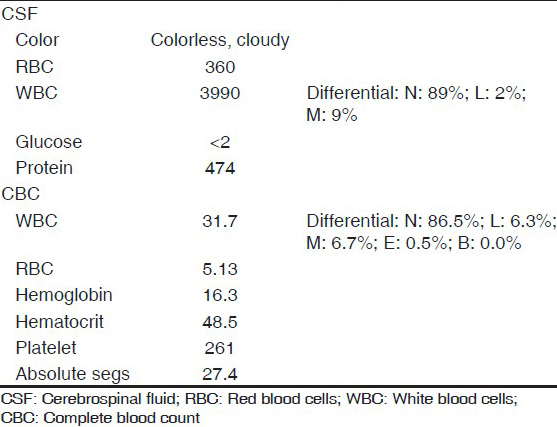
Vitals on admission were: BP 160 mmHg/108 mmHg; pulse 88 bpm, regular rate and rhythm; and temperature 100.4°F. He was initially lethargic but arousable. He had no nuchal rigidity, no tenderness on passive neck movement, other meningeal signs or focal cranial nerve deficits. The patient was unable to fully cooperate with the neurological exam but was able to move all extremities spontaneously. Shortly after the initial evaluation, his condition rapidly deteriorated, he became confused and incoherent and was intubated for airway protection. NCCT-head at that time showed increased attenuation of the proximal portion of the MCA and PCA, most prominently on the right side, but no significant mass effect or bleeding [Figure 1]. Lumbar puncture revealed elevated opening pressure (35.2 cm of the cerebrospinal fluid [CSF]). Gram stain of the CSF was consistent with bacterial meningitis [Table 1]. The patient was started on meningitis dose intravenous vancomycin, ceftriaxone, acyclovir and dexamethasone. Both blood and CSF culture later grew Streptococcus pneumoniae sensitive to ceftriaxone and penicillin. His condition deteriorated quickly thereafter, his BP dropped and he needed pressor support. He spiked temperature once (103.4°F), but was afebrile the rest of the hospital course. Patient's repeat NCCT-head done ~11 h postadmission showed increased density in the interpeduncular cistern (concerning for subarachnoid hemorrhage [SAH]), decreased ventricular volume and loss of gray–white matter differentiation [Figure 1]. The prominence of vascular territories persisted, but was obscured by both increasing edema and signal attenuation in the cisterns. The patient was given seizure prophylaxis with levetiracetam on suspicion of SAH. In the next 24 h, the patient was comatose without discernible brain stem function. The Tc99m Exametazime brain flow scan showed no intracerebral blood flow, both supra- and infratentorially [Figure 2].

- Axial noncontrast-enhanced computed tomography (NCCT)-head images through the lateral ventricles (a) and basal cisterns (b), taken soon after admission. Axial NCCT images through the lateral ventricles (c) and basal cisterns (d), ~11 h after admission. a and b: Loss of gray white matter differentiation and decreased ventricular volume in c compared with a, concerning for global edema/global ischemic event. b and d: Single arrowheads in b and d indicate the location of the hyperdense posterior cerebral artery; double arrowheads indicate the hyperdense middle cerebral artery sign. Note development of increased density in the intrapeduncular cistern in d not seen in b. There is also increased density in the posterior tentorium close to the calvarium in d
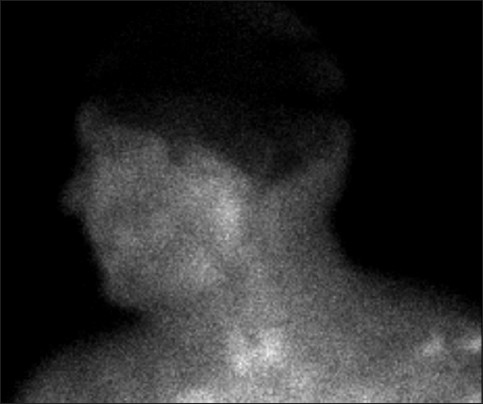
- Brain flow study, left lateral view after administration of 32.6 mCi of Technetium Tc99m Exametazime intravenously. There was no blood flow seen both supra-and infratentorially
Discussion
Fulminant Pneumococcal meningitis, presenting with altered mental status and headache but without fever or overt signs of meningeal irritation, suggests meningitis in an immunosupressed state.[12] The attenuation of MCA and PCA noted in the initial head CT scan (dense MCA/PCA sign) indicate that thrombosis of cerebral arteries occurred early in the clinical course. Lack of blood flow in the Tc99m Exametazime brain flow scan shortly after presentation and imaging findings of hyperdense large artery sign are compatible with thrombosis of the major cerebral large arteries. The patient did not have other factors known to be associated with hyperdense cerebral artery sign not associated with thrombosis, such as high hematocrit, diabetes, hypertension, HSV encephalitis, posttraumatic edema or prior injection of IV contrast media.[145]
The unusual presentation of extensive intraarterial thrombosis at the onset of this patient's symptoms suggests that it was caused by an acute local activation of the coagulation pathways. Pneumococcus, high-dose steroids and Non-Hodgkins lymphoma (NHL) cells can individually augment the expression of tissue factor, the evidence for which are (1) Pneumococcus has been shown to activate the coagulation system in Pneumococcal pneumonia locally[13] and systemically[14] via upregulation of tissue factor in alveolar macrophages, neutrophils and endothelial cells leading to the activation of the extrinsic pathway;[1516] (2) plasma tissue factor antigen is significantly increased in patients with malignant lymphoma (for review see[17]) which can lead to a propensity to activate the extrinsic pathway;[3] (3) this patient had undergone an allogenic sibling T-cell infusion 6 years earlier and was in immunologic symbiosis with his innate non-Hodgkin's lymphoma cells; (4) high-dose glucocorticoids can tip the balance toward sustained coagulation and platelet aggregation by influencing the tissue factor/extrinsic pathway.[18] Another study noted that subjects who were pretreated with glucocorticoids 12-144 h before endotoxin exposure had significantly higher levels of proinflammatory cytokines and were at an increased risk of coagulation.[19]
Increased attenuation of the basal cisterns and subarachnoid spaces in second NCCT of the head was most likely “pseudo-subarachnoid hemorrhage,” reported previously in pyogenic leptomeningitis and diffuse cerebral edema[20212223242526] because (1) the maximum Hounsfield unit (HU) in this patient was 28 (true SAH: 60-70 HU) and[27] (2) CSF collected within 24 h of onset of symptoms showed a WBC: RBC ratio of 11.08, consistent with a nontraumatic tap. Various mechanisms have been postulated in the causation of pseudo-SAH. These include (a) presence of toxins in meningitis leading to the breakdown of the blood–brain barrier, resulting in a leak of proteinaceous material into the subarachnoid space, (b) increased intracranial pressure and brain swelling causing narrowing of the subarachnoid spaces and displacement of CSF, leading to decrease in the low attenuation associated with CSF, (c) raised intracranial pressure causing an engorgement and dilation of the superficial venous structures causing increased blood to superficial venous structures and (d) cerebral edema leading to a decrease in attenuation of the brain parenchyma, leading to the appearance of increased differentiation between the CSF and the parenchyma.[2728]
This case report highlights the catastrophic consequences of the coexistence of factors that activate extrinsic pathways in patients with Pneumococcal meningitis. Very rapid neurological deterioration and the presence of hyperdense large arterial sign on CT scan are markers of this ominous presentation of Pneumococcal meningitis.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- The hyperdense posterior cerebral artery sign: A computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke. 2006;37:399-403.
- [Google Scholar]
- Radiologic-pathologic correlation of the hyperdense middle cerebral artery sign. A case report. Acta Radiol. 2001;42:467-9.
- [Google Scholar]
- Hyperdense middle cerebral arteries identified on CT as a false sign of vascular occlusion. AJNR Am J Neuroradiol. 1993;14:669-73.
- [Google Scholar]
- What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10:419-23.
- [Google Scholar]
- Peripheral symmetrical gangrene successfully treated with epoprostenol and tissue plasminogen activator. Lancet. 1986;2:1401-2.
- [Google Scholar]
- Symmetrical peripheral gangrene (purpura fulminans) complicating pneumococcal sepsis. Am J Surg. 1993;165:642-5.
- [Google Scholar]
- Cerebrovascular complications of bacterial meningitis in adults. Neurology. 1992;42:1497-504.
- [Google Scholar]
- Pneumococcal meningitis in adults: Spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126:1015-25.
- [Google Scholar]
- Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol. 1984;2:187-93.
- [Google Scholar]
- Advances in the management of central nervous system infections in the ICU. Crit Care Clin. 2006;22:661-94.
- [Google Scholar]
- Local activation of the tissue factor-factor VIIa pathway in patients with pneumonia and the effect of inhibition of this pathway in murine pneumococcal pneumonia. Crit Care Med. 2006;34:1725-30.
- [Google Scholar]
- Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol Med. 2009;15:438-45.
- [Google Scholar]
- Blood-borne tissue factor: Another view of thrombosis. Proc Natl Acad Sci USA. 1999;96:2311-5.
- [Google Scholar]
- Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22:401-4.
- [Google Scholar]
- High-dose glucocorticosteroids increase the procoagulant effects of OKT3. Kidney Int. 1994;46:1596-602.
- [Google Scholar]
- Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999-2006.
- [Google Scholar]
- Subarachnoid contrast enhancement after spinal angiography mimicking diffuse subarachnoid hemorrhage. AJR Am J Roentgenol. 1998;170:503-5.
- [Google Scholar]
- Acute purulent leptomeningitis mimicking subarachnoid hemorrhage on CT. J Comput Assist Tomogr. 1994;18:126-8.
- [Google Scholar]
- Increased density of tentorium and falx: A false positive CT sign of subarachnoid hemorrhage. Can Assoc Radiol J. 1986;37:243-7.
- [Google Scholar]
- Pseudo-subarachnoid hemorrhage: A rare neuroimaging pitfall. Can J Neurol Sci. 1999;26:57-9.
- [Google Scholar]
- Pseudo-subarachnoid hemorrhage: A potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol. 2003;24:254-6.
- [Google Scholar]
- CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol. 1998;28:222-5.
- [Google Scholar]
- Cryptococcal meningitis presenting as pseudosubarachnoid hemorrhage. South Med J. 2008;101:1255-7.
- [Google Scholar]
- Pseudosubarachnoid hemorrhage in a 42-year-old male with meningitis. Radiol Case Rep. 2011;6:470.
- [Google Scholar]






