Translate this page into:
Hydrocephalus Associated with Large Vestibular Schwannoma: Management Options and Factors Predicting Requirement of Cerebrospinal Fluid Diversion after Primary Surgery
Address for correspondence: Dr. A. R. Prabhuraj, Department of Neurosurgery, National Institute of Mental Health and Neurosciences, Bengaluru - 560 029, Karnataka, India. E-mail: drprabhuraj@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
Obstructive hydrocephalus (HCP) related to vestibular schwannoma occurs in large tumors compressing the fourth ventricle. Symptoms related to HCP are expected to alleviate after resection of the tumor and decompression of the cerebrospinal fluid (CSF) pathways. However, some patients may require permanent cerebrospinal diversion even after surgery due to persistent HCP. In this study, the authors try to find out the factors associated with the requirement of CSF diversion after vestibular schwannoma surgery in cases of persistent HCP.
Materials and Methods:
This was a retrospective study involving 193 cases of vestibular schwannoma operated between 2010 and 2013 in our institute. Cases that underwent ventriculoperitoneal (VP) shunts before surgery were compared to cases which were operated directly. In cases where vestibular schwannomas were operated without prior VP shunts, factors which were associated with persistent postoperative HCP were analyzed.
Results:
Comparing the group who underwent direct surgery to the group who underwent VP shunt before definitive vestibular schwannoma surgery, the facial nerve preservation rates and surgical morbidity rates were comparable. In cases who underwent direct surgery, 10 out of 75 patients required postoperative permanent CSF diversion. Older age, male gender, duration of symptoms, larger tumor size, solid lesions, severe HCP, and clinical features of HCP were associated with postoperative requirement of CSF diversion but were not statistically significant. The most significant factor that correlated with the need for additional HCP treatment was the presence of postoperative hematoma of volume >10cc.
Conclusions:
Primary tumor removal is the optimal treatment for vestibular schwannoma associated with HCP. Postoperative hematoma may warrant close observation as these patients are at an increased risk of persistence of HCP.
Keywords
Acoustic neuroma
hydrocephalus
large vestibular schwannoma
ventriculoperitoneal shunt
INTRODUCTION
Vestibular schwannomas (VS) are benign tumors of the vestibular nerve that produce unilateral sensorineural hearing loss and tinnitus, as they enlarge they protrude into the cerebellopontine angle (CPA) to compress the cerebellum, fourth ventricle, brainstem, seventh and fifth cranial nerves.[1] Large VS may produce obstructive hydrocephalus (HCP) by compressing the fourth ventricle. This will produce symptoms and signs of intracranial hypertension such as headache, nausea and vomiting, depressed level of consciousness, and papilledema.
The incidence of HCP associated with vestibular schwannoma ranges from 3.7% to 42%.[23456] Communicating HCP is more frequent (61%–87.2%) than obstructive HCP in patients with VS.[78] Communicating HCP is reported to result from a number of mechanisms, either alone or in combination, including obstruction of cerebrospinal fluid (CSF) absorption at the arachnoid granulation by protein leakage from the tumor; a decrease in intracranial compliance due to adhesion of the subarachnoid space; meningeal adhesion due to minor hemorrhage from the tumor; and CSF malabsorption, likely due to tumor cells and high fibrinogen concentration in the CSF.[6910]
When there is obstructive HCP, the symptoms related to HCP are expected to alleviate after resection of the tumor and decompression of the CSF pathways.[4] Although there is a lack of evidence in literature, it is true that surgery in patients with HCP and increased intracranial pressure (ICP) is presumably more challenging[489] and can lead to worse outcomes or higher complication rates ranging from 5.1% to 36%.[23] Hence, some surgeons prefer initial treatment of the HCP with placement of a ventriculoperitoneal (VP) shunt before surgery although it can be managed with an external ventricular drain in the perioperative period. Recent evidence, however, indicates that complete removal of CPA tumors will result in resolution of HCP without the need to insert a permanent shunt[23511] and is currently considered as preferred mode of treatment. Although direct surgery avoids permanent CSF diversion in many patients, the ventricle size is to be monitored postoperatively as HCP can persist or reappear postoperatively, needing additional treatment.
In this study, we reviewed all the patients with unilateral VS with obstructive HCP presenting with features of raised ICP. Some surgeons preferred to do a VP shunt for their cases before tumor resection and some operated without any CSF diversion. In this study, we looked into factors which lead to persistent HCP after VS surgery who required a VP shunt later.
MATERIALS AND METHODS
This is a retrospective study conducted in the Department of Neurosurgery, National Institute of Mental Health and Neurosciences. All patients with unilateral VS who were operated between 2010 and 2013, were included in the study. Medical records of all the included patients were reviewed including the presenting symptoms, duration, type of surgery, and management of HCP; postoperative complications and outcome at follow-up. The radiological data were reviewed for severity of the HCP, tumor size and volume, and postoperative extent of resection and alleviation of HCP. HCP was graded by assessing the ratio of the maximal width of the frontal horns to the maximal diameter of the inner table of the cranium at the same level[46] (Evan's ratio). Obstructive HCP was defined by a disproportionally small fourth ventricle in relation to the lateral and third ventricles. There were no cases of communicating HCP in this study. HCP was graded as mild if the ratio was 30%–39%, moderate if the ratio was 40%–45%, and severe if it was ≥46%. Patients who had bilateral tumors, recurrent tumors, and patients who had undergone primary gamma knife radiosurgery were excluded from the study.
Among the included patients, those presenting with HCP were selected and were divided into two groups, based on whether they received primary treatment for HCP or underwent primary surgery for resection of VS. Among the patients who underwent primary resective surgery, number of patients who needed additional treatment for persistent HCP postoperatively were identified. In case the symptoms of HCP did not resolve postoperatively, or clinical deterioration occurred later, with a corresponding persisting dilation or increase in width of the ventricles, a ventriculoperitoneal shunt was done. Different factors assessed at analysis were patient-related factors such as age at initial presentation, sex, symptoms, duration of symptoms, duration and severity of raised ICP. Tumor-related factors such as tumor size (largest extrsmeatal diameter),[12] volume, shape internal structure (solid, cystic, or solid-cystic), and extension classified as per the Hannover tumor extension grading system[13] were noted.
All patients underwent surgery in the lateral position, with the head flexed anteriorly and rotated contralaterally with lateral flexion. A standard lateral retrosigmoid suboccipital approach and decompression were done with multimodality monitoring in a standard manner.[1314] All patients were operated with the aim to achieve complete tumor removal, with preservation of all neurovascular structures and neurological functions. Postoperative facial nerve function was assessed using House–Brackmann grading system,[15] and functional hearing was graded according to the new Hannover classification.[16] Operative morbidity like severe cerebellar bulge requiring partial removal of cerebellum, CSF leaks, and prolonged ventilator support due to lower cranial nerve palsy was noted.
Statistical analysis was done with commercially available software “(SPSS 20, IBM, Chicago)”, Mann–Whitney U-test for continuous variables and categorical variables by Fisher's exact test.
RESULTS
A total of 193 vestibular schwannomas were operated during the study period. Out of these, 145 (75%) patients presented with imaging showed obstructive HCP and the remaining 48 patients (25%) had no HCP. Of the 145 patients with HCP, 70 (48.3%) underwent VP shunt before VS surgery and 75 patients (51.7%) underwent primary surgery without any prior VP shunt [Figure 1]. The decision to do VP shunt was done either because of the increased waiting period for definitive surgery or because of some surgeon's preference. The definitive VS surgery was done after a median of 14 days (3–120 days) after shunt surgery. Some surgeons opted to do surgery even in cases where HCP was present with or without raised intracranial features, especially during periods when waiting period for surgery was less. Nineteen (25.3%) patients underwent surgery with placement of perioperative external ventricular drainage (EVD) with the intention to remove it after tumor decompression.
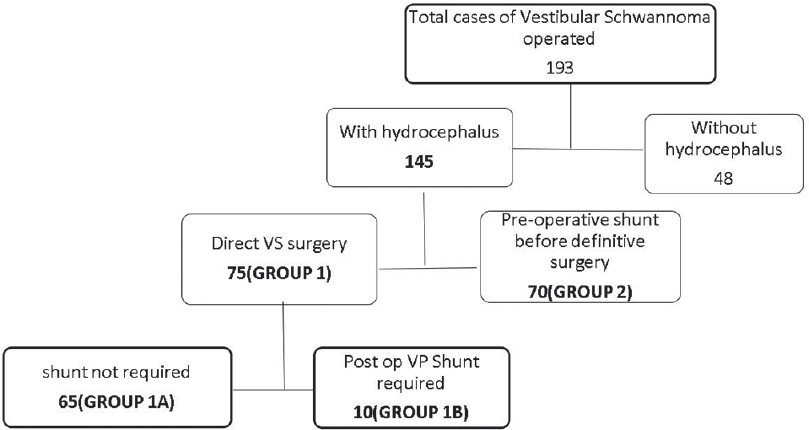
- Flow chart showing the management of all cases of vestibular schwannomas
Among the 75 patients who underwent direct VS surgery, 10 patients (13.3%) needed further treatment for persistent postoperative HCP and the remaining 65 patients (86.7%) needed no additional treatment at a median follow-up period of 6 months. For patients who required postoperative CSF diversion, VP shunts were done at a mean follow-up period of 8 ± 4.2 days. Four of the patients who required subsequent CSF diversion had a preoperative EVD which was converted to VP shunt at 7, 6, 4, and 10 days after VS surgery, respectively.
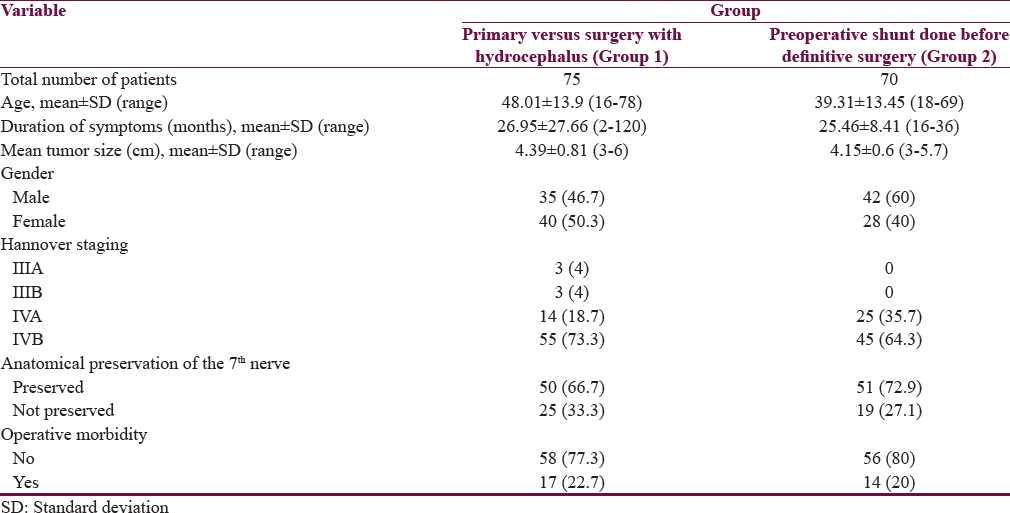
Comparing the group who underwent direct surgery (Group 1) to the group who underwent shunt before definitive VS surgery (Group 2), all the demographic parameters were comparable [Table 1]. However, all the Group 2 patients had Hannover Grade IV compared to 8% of Group 1 who were Grade III (P = 0.011). The facial nerve preservation rate was 73% in Group 2 compared to 66.7% in Group 1 (P = 0.418). Surgical morbidity was 22.7% in Group 1 compared to 20% in Group 2 (P = 0.696).
Among the 75 patients who underwent direct surgery, 65 patients (Group 1A) needed no further treatment for HCP as patients’ clinical symptoms of raised ICP emanated after surgery; but, 10 patients (Group 1B) required a VP shunt postoperatively for HCP. Shunt surgery was done after a mean of 8 ± 4.2 days after VS surgery. Four of the patients who required subsequent shunt showed features of raised ICP after blocking the EVD and hence required to be converted to VP shunt at 7, 6, 4, and 10 days after VS surgery, respectively [Table 2].
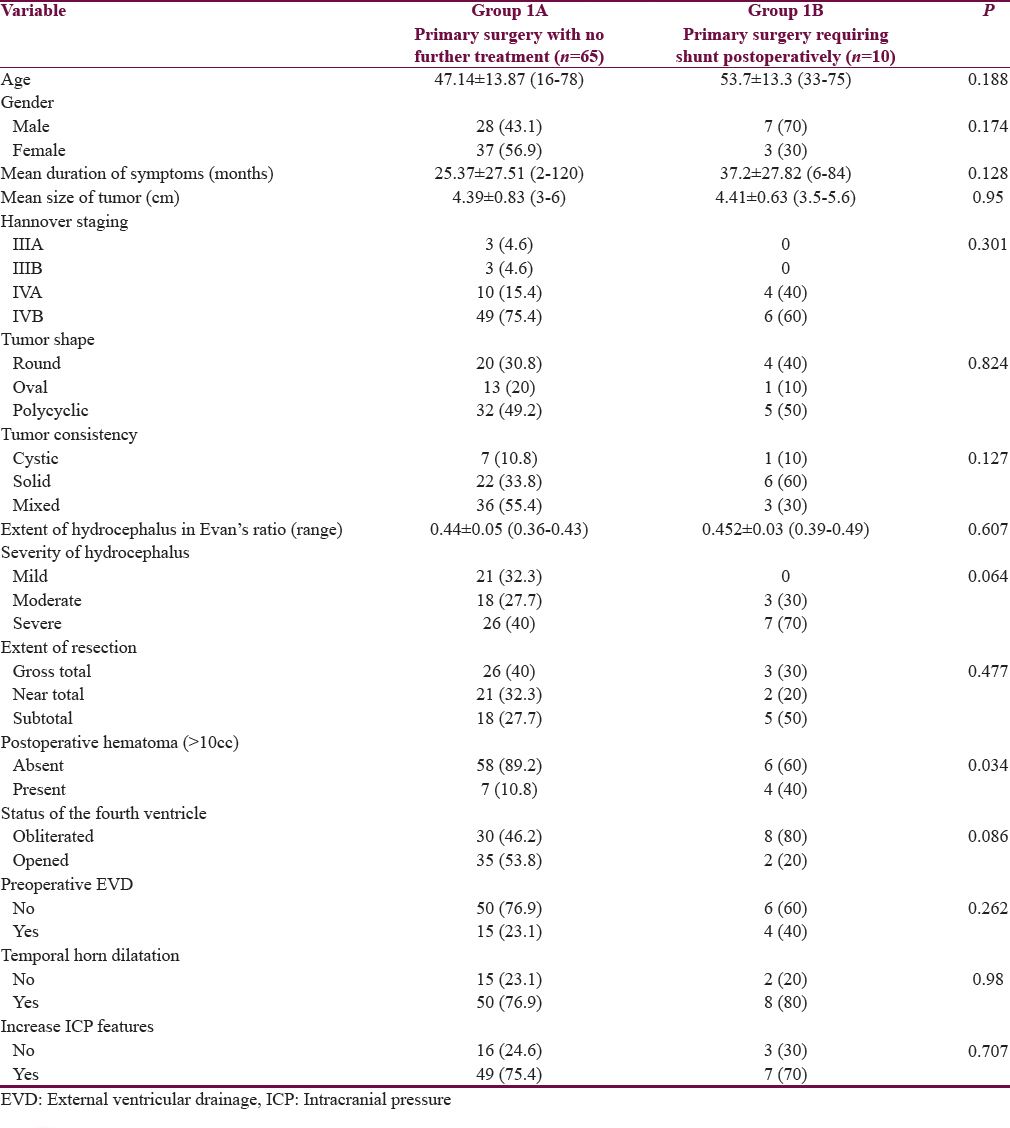
Patients who required a postoperative CSF diversion were older (mean 53.7 vs. 47.1 years), males, had more duration of symptoms (mean 25.37 vs. 37.2 months), larger tumor size (mean 4.41 cm vs. 4.39), more solid tumors, more severe HCP with obliteration of the fourth ventricle and had clinical features of HCP, compared to patients who did not require postoperative CSF diversion. However, these variables did not have statistical significance. The only factor which reached a statistical significance to predict the requirement of postoperative CSF diversion was development of postoperative tumor bed hematoma. Eleven patients out of 75 had postoperative tumor bed hematoma, out of which, 4 required a VP shunt postoperatively [Figures 2 and 3], compared to 6 patients requiring shunt out of 64 patients who did not develop hematoma (P = 0.034). Out of the four patients who had hematoma and required a postoperative VP shunt, one of them required reexploration of surgery to evacuate tumor bed hematoma and required a CSF diversion 7 days later.
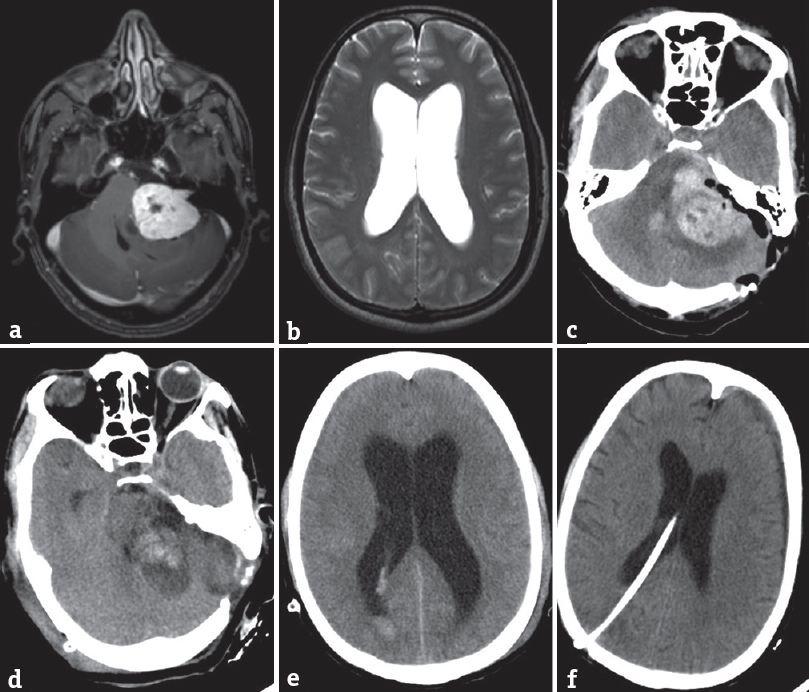
- (a) A preoperative T1-weighted postgadolinium image showing a large left vestibular schwannoma. (b) T2-weighted image showing hydrocephalus. (c) Postoperative computer tomogram showing tumor bed hematoma which was evacuated, (d and e) showed a persistent hydrocephalus 5 days after surgery with patient having clinical signs of raised intracranial pressure. (f) A scan done 5 days later with resolution of hydrocephalus due to a ventriculoperitoneal shunt
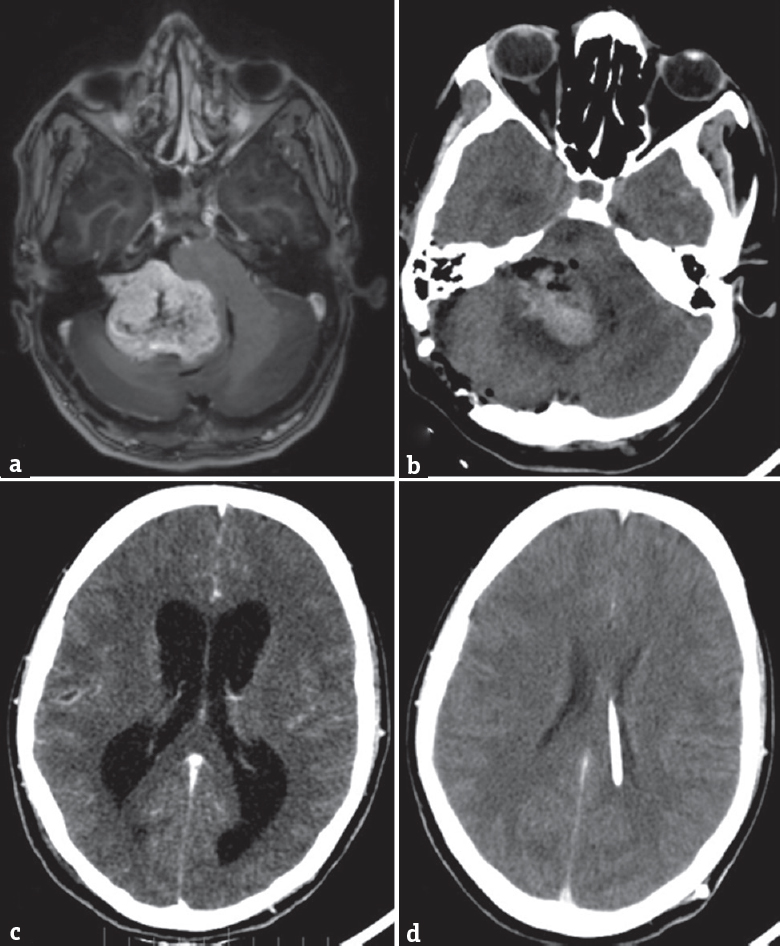
- (a) A large right vestibular schwannoma on a T1-weighted postgadolinium scan. (b) Postoperative tumor bed hematoma after surgery causing persistent ventriculomegaly as shown in c. (d) Decompressed ventricles by a ventriculoperitoneal shuntwhy
DISCUSSION
The incidence of HCP in cases of VS has been published to be varying from 3.7% to 42%.[5] The wide variation is likely due to the inclusion of different sizes of tumor in patients with different ages. It has also been told that features of communicating HCP are more common up to 61%–87.2%.[7] As most of the studies described earlier had included both features of obstructive and nonobstructive HCP in their series,[457810] this series may not be directly comparable to them. As most of the cases in our series were large, Hannover grade IV tumors and were referred from elsewhere, it is the probable reason why our patients had obstructive HCP more often. The overall mean tumor size of all patients included in this study was 4.3 cm. Another factor is the poor awareness of such conditions in patients from developing countries.
Although there is some evidence that VS cases with HCP can be managed more often directly without any prior CSF diversion,[4510] these studies included cases which had features of normal pressure HCP due to nonobstructive HCP. There is a common acceptance that large tumors with obstructive HCP can cause operative morbidity due to cerebellar bulge, venous bleeding, difficulty achieving hemostasis, and need of retraction. It has also been previously stressed that sitting position may have less of these operative difficulties,[17] and a perioperative external ventricular drain may increase safety in cases with raised ICP. The recommended crucial action that allows the surgeon to control the ICP is to open the cerebellomedullary cistern early and drain sufficient CSF. Craniotomy can be extended down and laterally and dural incised basally, to elevate the cerebellum and access the cisterns.[17]
In our study, almost an equal number of patients underwent either direct VS surgery or a VP shunt followed by surgery. We noted that in patients who were operated primarily for VS, the anatomical preservation of seventh nerve and operative morbidity was comparable to those patients who were operated following treatment for HCP, and there was no statistical difference noted upon between the two groups; hence, it can be concluded that primary removal of tumor for VS with coexisting HCP is optimal treatment option. Although in cases where there is suspected severe raised ICP, a perioperative EVD may increase safety and avoid operative morbidity.
One can argue that in many of the cases, a VP shunt could have been differed and a direct surgery could be done. Because of a long waiting list, some surgeons prefer a VP shunt at least a few weeks before definitive surgery. This study highlights the necessity of such practice.
In this study, 86.6% of the 75 patients who were treated primarily with surgery needed no further treatment of HCP which was comparable to results of other studies which ranged from 75% to 90%.[4510] Several factors have been identified earlier that could predict the persistence of HCP in postoperative period. These include mean tumor size,[4] duration of disease,[10] inhomogeneous polycyclic tumor, and presence of severe HCP before surgery.[5] In our cases, older age, male gender, duration of symptoms, larger tumor size, solid lesions, severe HCP, and clinical features of HCP were associated with postoperative requirement of CSF diversion but were not statistically significant.
The most significant factor that correlated with the need for additional HCP treatment was the presence of postoperative hematoma of volume >10cc. The possible explanation for the evolution of HCP in patients with postoperative hematoma could be due to seepage of blood into the cisternal spaces. Hence, in such patients, careful clinical and radiological observation is warranted, especially during the early postoperative period. Postoperative hematoma and cerebellar edema may lead to the fourth ventricle obliteration which may lead to requirement CSF diversion even after evacuation of hematoma.
CONCLUSIONS
Primary tumor removal is the optimal treatment for vestibular schwannoma-associated HCP. When there is severe HCP, a perioperative EVD is a valid option. Postoperative hematoma may warrant close observation as these patients are at an increased risk of persistence of HCP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Management of 1000 vestibular schwannomas (acoustic neuromas): Clinical presentation. Neurosurgery. 1997;40:1-9.
- [Google Scholar]
- Management of hydrocephalus resulting from acoustic neuromas. Otolaryngol Head Neck Surg. 1993;109:1020-4.
- [Google Scholar]
- Evolution of the management of hydrocephalus associated with acoustic neuroma. Laryngoscope. 1996;106(2 Pt 1):204-6.
- [Google Scholar]
- Management of hydrocephalus associated with vestibular schwannoma and other cerebellopontine angle tumors. Neurosurgery. 2001;48:1246-53.
- [Google Scholar]
- Hydrocephalus associated with vestibular schwannomas: Management options and factors predicting the outcome. J Neurosurg. 2011;114:1209-15.
- [Google Scholar]
- Etiopathological factors related to hydrocephalus associated with vestibular schwannoma. Neurosurgery. 2007;61:1186-92.
- [Google Scholar]
- Prevalence of hydrocephalus in 157 patients with vestibular schwannoma. Neuroradiology. 2005;47:344-51.
- [Google Scholar]
- Clinical and neuroimaging characteristics of hydrocephalus associated with vestibular schwannoma. J Neurosurg. 2003;98:1188-93.
- [Google Scholar]
- Hydrocephalus in the patient with acoustic neuroma. Otolaryngol Head Neck Surg. 1992;107:35-9.
- [Google Scholar]
- Hydrocephalus associated with vestibular schwannomas: Perioperative changes in cerebrospinal fluid. Acta Neurochir (Wien). 2013;155:1271-6.
- [Google Scholar]
- Case of hydrocephalus associated with vestibular schwannoma, treated by tumor removal. No Shinkei Geka. 2006;34:391-5.
- [Google Scholar]
- New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24:642-8.
- [Google Scholar]
- Management of 1000 vestibular schwannomas (acoustic neuromas): Surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11-21.
- [Google Scholar]
- Suboccipital retrosigmoid approach for removal of vestibular schwannomas: Facial nerve function and hearing preservation. Neurosurgery. 2005;56:560-70.
- [Google Scholar]
- Management of 1000 vestibular schwannomas (acoustic neuromas): Hearing function in 1000 tumor resections. Neurosurgery. 1997;40:248-262.
- [Google Scholar]






