Translate this page into:
Higher sensory processing sensitivity, introversion and ectomorphism: New biomarkers for human creativity in developing rural areas
Address for correspondence: Dr. Fidias E. Leon-Sarmiento, Smell and Taste Center, 5 Ravdin Pavilion 3400, Spruce Street, Philadeplhia, PA 19104, USA. E-mail: feleones@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The highly sensitive trait present in animals, has also been proposed as a human neurobiological trait. People having such trait can process larger amounts of sensory information than usual, making it an excellent attribute that allows to pick up subtle environmental details and cues. Furthermore, this trait correlates to some sort of giftedness such as higher perception, inventiveness, imagination and creativity. We present evidences that support the existance of key neural connectivity between the mentioned trait, higher sensory processing sensitivity, introversion, ectomorphism and creativity. The neurobiological and behavioral implications that these biomarkers have in people living in developing rural areas are discussed as well.
Keywords
Developing rural areas under conflict
highly sensitive person
perception
inhibition
Introduction
The highly sensitive (HS) trait is a recently proposed human trait, found in up to 20 % of the population,[1] which allows to process information deeper than usual.[2] This trait makes HS people more prone to arousal, especially after exposure to sense stressors such as bright lights, loud noise, strong smells as well as dense and chaotic environments.[1] This people may process, at the same time, larger amounts of sensory information than usual, making this trait an excellent model to pick up subtle environmental details and cues. However, they feel easily worn out, overwhelmed and exhausted because they sense every single detail while interacting with their environment.[34] To recover from such attainable sensory overload, these individuals require more quiet time daily to be alone, as well as additional longer sleep times than those without the HS trait.[3–5] Further, the HS trait correlates with higher perception, consciousness, inventiveness, imagination and creativity.[1] Therefore, a relationship between higher sensory processing sensitivity, introversion, ectomorphism and creativity is proposed, which may have strong neurobiological and behavioral implications in developing rural areas, mostly in those under social conflict.
Animal Studies
Experiments in animals have demonstrated that dominant individuals differ from non-dominants. The trait for dominance is the lack of fear or higher confidence while for submissiveness is fear or lower confidence.[6] Further, the behavior of aggressive males is, mainly, controlled by intrinsic factors, whereas the non-aggressive ones rely more on external factors.[7–12]
Germane to this, dominant rodents[7] forming quickly a routine are not influenced by environmental factors, such as maze configuration changes. In fact, after hours without receiving food, the dominant male mouse looks for food impulsively through the maze, without reacting to the novel environment, despite the location of food is changed. Submissive rodents, on the other hand, are strongly influenced by minor changes.[8] They seem more cautious and less impulsive than the dominant ones. Moreover, they wait for more minutes than dominant animals before approaching foods in new locations.[11]
It is also accepted that male mice and rats[7] born in unstable, unpredictable environments, similar to those found in developing rural areas, would develop submissiveness behavior, at an early age. It helps them to save energy developing smaller and immature body frames paralleling delayed secondary male characteristics.[13] Since submissive animals do perceive subtle changes, it allows them to easily adapt to rapidly changing environments.[712–14] On the other hand, stable environments, such as those found in developed urban areas with no social conflicts, would be more suitable for animals who do not sense subtle changes, indeed the dominant ones.[71214]
In the same line, Henry and Stephens[11] were the first who suggested that individual variations in aggression might be an expression of behavioral mismatch with environmental challenges, which was later demonstrated using different force-choice paradigms.[11] For example, in the defensive burying experiment,[11] animals were confronted with an electrified probe inserted into their home cage. In response to a brief contact with the shock probe, the animal actively buried the probe with the bedding of their cage (dominant type), or showed immobility and passively avoided the electrified probe (submissive type).
Other studies have also found that behavioral measures, metabolic rate, immunological function and response to stressful environments differed between submissive and dominant animals, adding support to our view.[1516]
Human Studies
The above-described behavioral characteristics in animals resemble those found in humans[61718] [Figures 1 and 2]. Highly reactive human babies have shown higher heart rates since fetal age compared to controls.[6] Moreover, these babies display stronger motor responses to sensory stimulation such as, noise and odors.[6] When they are placed in unknown environment, they tend to cry more than usual. They also show a high heart rate above standard values when confronted to new situations.[6] In the same vein, higher levels of salivary cortisol were found in inhibited children, tested at home or in the laboratory.[18] And, they show more activity in the right brain hemisphere and the vegetative nervous system.[6] Lower threshold of reactivity to unfamiliar places is reflected as limbic overactivity, especially the amygdala and its projections to the hypothalamus.[618] Of remark, higher levels of norepinephrine which improves the signal to noise ratio in different brain areas including amygdala, may lower chemosensory thresholds.[18]

- Schematic representation of ectomorphic, mesomorphic, and endomorphic constitution in western medicine, which is similar to the Vata, Pitta and Kaphasomatotypes described in ancient traditional Indian medicine
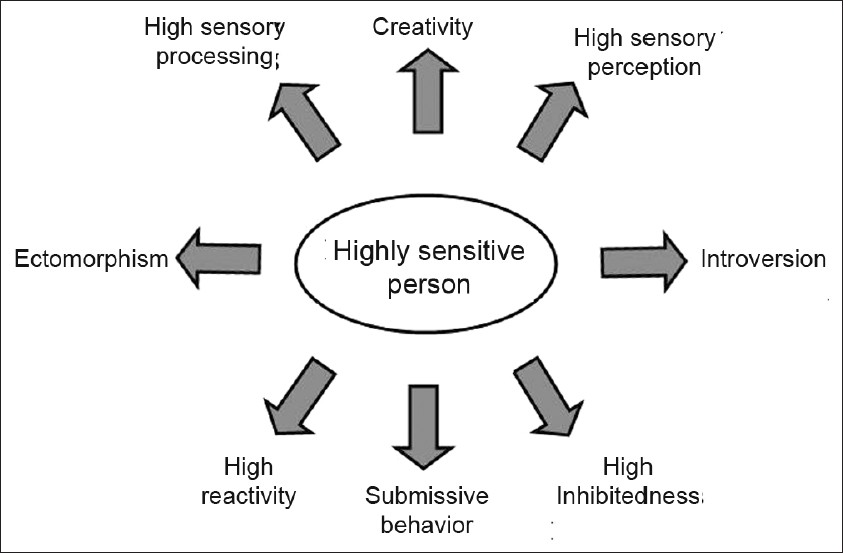
- Schematic representation of the predisposition and tendencies associated to the highly sensitive person that have to be considered in behavioral rural practice and research
It has been suggested that the probability to be born dominant or submissive depends on the genetics as well. Interestingly, inhibited children or high reactive babies are more likely to possess a tall, lean body mass, narrow face and light blue eyes.[61718] This striking similarity with ectomorphic constitution also suggest that it is the most likely phenotype for HS persons.[1319–21] In line with this, it is known that highly reactive and irritable babies become inhibited children,[6] , and display the HS trait at teenage and adult times.[13] It suggests that this trait is inherited becoming behaviorally evident late in life.
Current Facts
Recent studies have found a link between the HS trait and the higher sensory processing sensitivity (SPS) trait.[1722] For instance, functional magnetic resonance imaging (fMRI) of the visual system demonstrated HS trait differences when making context-dependent vs. context-independent judgments of simple visual stimuli.[17] . Another study used also fMRI to investigate subtle visual stimulation effects in higher SPS people. Greater activation was found in right claustrum, left occipitotemporal, bilateral temporal, and medial and posterior parietal brain circuits as well as right cerebellum.[22] These results provide further evidence of specific neural involvement associated with HS trait, as well as higher SPS; they also support the existence of heightened sensory processing in these individuals, in particular during visual imagery.
Moreover, recent neurobiological investigations linked temperaments and creativity with biological and psychophysical characteristics, such as sympathetic tone variations in the cardiovascular system,[6] as well as trigeminal,[23] tactile[24] and chemosensory thresholds,[1825] as well as testosterone level.[26] These findings add support to the notion that sensory capabilities may provide a filter through which we perceive the world that may influence the picture we receive from it.[25] Since enhanced sensitivity of sensory and motor brain areas were related to neuroticism;[2527] it could be the case that the perceived environment influences perception and is, therefore, able to modulate it as well, mostly in creative people.[25]
Of note, highly creative people are more prone to experience anxiety.[28–30] They are also more vulnerable to suffer from mood and mental disorders.[28–31] In line with this view, schizophrenia spectrum disorders and alcoholism tendencies are more often found in highly sensitive people.[28–31] Of remark, the HS trait and ectomorphism are two additional co-factors for vulnerability in a current creativity and psychopathology model.[2832]
Concluding Facts
Some of the aforementioned attributes are also considered in Theotherapy. This is a novel behavioral approach based on ancient descriptions done in the Middle East, which was introduced in Latin America by Chamorro et al.[33] Similarly, the traditional medicine from India commented on the so-called Vata somatotype.[1321] It possesses higher sensory processing sensitivity, introversion and creativity-like characteristics as well as some of the features described for the submissive, ectomorphic type[1920] reported in current westernized medicine.
Such ectomorphic type described in ancient times resembles the so-called youngster body frame,[13192034] not fully developed. It is found in people having long and thin muscles / limbs and low fat storage, which is usually referred to as slim. Due to their physical frame, they are not suitable for heavy physical work or exhausting long exercises.,[1321] and require more sleep hours compared to other body types to get “recharged”. This body type [Figure 1] displays more cautious and submissive behavior; it is more sensitive, artistic and perceptive to the environment and introverted.[18–20] If in balance, they are vibrant, enthusiastic, clear and alert mind, flexible, imaginative, sensitive, talkative, and quick to respond;[1321] If out of balance, they tend to be worried, feared, anxious and depressed, with restless mind, light sleep, tendency to overexert, fatigued, constipated and underweight, among other characteristics.
In line with these facts, an ectomorphic trait has to be found at higher prevalence in developing rural areas, mostly those under conflict. And, some evidence for such directional selection is provided by recent studies. Approximately, 20% of the population is highly sensitive, non-aggressive or submissive, driven by heighten sensory processing sensitivity and higher creativity.[1] The remainder of the population presents very low sensory processing thresholds, and it is not as creative as the other 20% of people commented above. It might be due to the better life style that is more likely to be found in stable environments,[35] among other variables, such as the gender issue;[36] however, more work is needed on this. Therefore, investigations that explicitly show that submission, introversion, hipersensitivity[37] ectomorphism and creativity is the dominant behavioral link in developing rural areas under social conflicts, some of which are more prone to experience environmental adversities as well, are worth examining.
Acknowledgment
The guidance and support received from Dr. Richard L. Doty is highly appreciated by Dr. Fidias E. Leon-Sarmiento. Dr. Carlos V Rizzo-Sierra thanks the kind support received from Dr. Flor A Vivas and Dr. Nestor Arias.
Source of Support: Dr. Fidias E. Leon-Sarmiento was supported by a grant from the Department of Defense, USA No. W81XWH-09-1-0467
Conflict of Interest: None declared.
References
- The highly sensitive person (HSP), how to thrive when the world overwhelms you 2003
- Memphis, TN: Poster presented at the annual meeting of the Society for Personality and Social Psychology; 2007. The personality/temperament trait of high sensitivity: fMRI evidence for independence of cultural context in attentional processing
- [Google Scholar]
- Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol. 1997;73:345-68.
- [Google Scholar]
- High sensitivity as one source of fearfulness and shyness: Preliminary research and clinical implications. In: Schmidt LA, Schulkin J, eds. Extreme fear, shyness and social phobia: Origins, biological mechanisms, and clinical outcomes. New York: Oxford University Press; 1999. p. :251-272.
- [Google Scholar]
- The long shadow of temperament. Washington DC: U.S. Library of Congress; 2004.
- Individual differences in behavioural reaction to a changing environment in mice. Behaviour. 1987;100:105-22.
- [Google Scholar]
- Evolutionary emergence of responsive and unresponsive personalities.Evolutionary emergence of responsive and unresponsive personalities. Proc Natl Acad Sci U S A. 2008;105:15825-30.
- [Google Scholar]
- For better and for worse: Differential susceptibility to environmental influences. Curr Dir Psychol Sci. 2007;16:300-4.
- [Google Scholar]
- Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307-21.
- [Google Scholar]
- Flexibility in animal signals facilitates adaptation to rapidly changing environments. PLoS One. 2011;6:1-4.
- [Google Scholar]
- Ayurvedic genomics, constitutional psychology and endocrinology: The missing connection. J Altern Complement Med. 2011;17:1-4.
- [Google Scholar]
- Consistent individual differences in early exploratory behavior of male great tits. Anim Behav. 1994;48:1113-21.
- [Google Scholar]
- Aggressive and non-aggressive personalities differ in oxidative status in selected lines of mice (Mus musculus) Biol Lett. 2008;4:119-22.
- [Google Scholar]
- Neuroendocrine and neurochemical impact of aggressive social interactions in submissive and dominant mice: Implications for stress-related disorders. Int J Neuropsychopharmacol. 2009;13:361-72.
- [Google Scholar]
- Temperament trait of sensory processing sensitivity moderates cultural differences in neural response. Soc Cogn Affect Neurosci. 2010;5:219-26.
- [Google Scholar]
- The Varieties of human physique: An introduction to constitutional psychology. New York: Harper; 1940.
- Atlas of men. New York: Macmillan; 1970.
- Ayurveda, nature's medicine 2001
- The trait of sensory processing sensitivity and neural responses to changes in visual scenes. Soc Cogn Affect Neurosci. 2011;6:38-47.
- [Google Scholar]
- Functional neurological evaluation of the brainstem. Part I: the blink reflex. Iatreia. 2009;22:372-81.
- [Google Scholar]
- Abnormal tactile discrimination and somatosensory plasticity in familial primary hyperhidrosis. Neurosci Lett. 2008;441:332-4.
- [Google Scholar]
- Agreeable smellers and sensitive neurotics–correlations among personality traits and sensory thresholds. PLoS One. 2011;6:1-9.
- [Google Scholar]
- Motor cortex excitability correlates with an anxiety-related personality trait. Biol Psychiatry. 2001;50:377-82.
- [Google Scholar]
- Creativity and psychopathology: A shared-vulnerability model. Can J Psychiatry. 2011;56:144-53.
- [Google Scholar]
- A meta-analytic investigation of the relationship of state and trait anxiety to performance on figural and verbal creative tasks. Pers Soc Psychol Bull. 2011;37:269-83.
- [Google Scholar]
- Decreased latent inhibition is associated with increased creative achievement in high-functioning individuals. J Pers Soc Psychol. 2003;85:499-506.
- [Google Scholar]
- Highly sensitive trait and ectomorphism: Another link on creativity and psychopathology. Can J Psychiatry. 2011;56:702-3.
- [Google Scholar]
- Amor y violencia: Otro coctel Neuropatológico en el siglo XXI. Salud Uninorte. 2009;25:350-361.
- [Google Scholar]
- EGLN1 involvement in high-altitude adaptation revealed through genetic analysis of extreme constitution types defined in Ayurveda. Proc Natl Acad Sci U S A. 2010;107:18961-6.
- [Google Scholar]
- Electromagnetic, hypersensitivity: evidence for a novel neurological syndrome. Int J Neurosci. 2011;121:670-6.
- [Google Scholar]






