Translate this page into:
GeneXpert: A Rapid and Supplementary Diagnostic Tool for Tuberculous Meningitis, Experience from Tertiary Neurocenter
Veena Kumari Haradara Bahubali, MD Department of Neuromicrobiology, National Institute of Mental Health and Neurosciences Hosur Road, Bengaluru, Karnataka 560029 India drveenas2002@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Tuberculous meningitis (TBM) is a highly lethal form of central nervous system tuberculosis (CNS TB) that causes high mortality and morbidity in children and adults. Nonspecific clinical presentation and fewer TB bacilli challenge clinicians resulting in delays in diagnosis and treatment.
Aim This study aimed to evaluate the utility of GeneXpert alone and in combination with culture using 1 mL of cerebrospinal fluid (CSF) in a volume constraint situation.
Methods A total of 125 clinically confirmed TBM and 110 non-TBM cases, comprised of both infectious and noninfectious diseases. were included in the study. Patient details including clinical signs and symptoms, CSF, and imaging data were collected from the case records. CSF samples were obtained from all the patients and were tested by the mycobacterial culture method and GeneXpert test. The performance of both the tests was statistically calculated and reported in the form of sensitivity and specificity.
Results Out of 125 TBM cases, 40 were detected positive by culture and 26 by GeneXpert. All 110 non-TBM cases were identified negative by both methods. The sensitivity and specificity of GeneXpert in comparison with culture were 27 and 100%, respectively. The culture was found to be more sensitive (32%) than GeneXpert. But the assay was able to detect a considerable number of clinically confirmed culture-negative TBM cases.
Conclusion GeneXpert is a rapid test and including this as an adjunctive test along with the culture in routine clinical practice can improve the diagnosis of TBM in volume constraint scenario.
Keywords
tuberculous meningitis diagnosis
Mycobacterium tuberculosis
cerebrospinal fluid
GeneXpert Mycobacterium tuberculosis/RIF
Introduction
Tuberculosis (TB) is a pulmonary infection caused by Mycobacterium tuberculosis, which can also cause extrapulmonary TB (EPTB).1 In 2019, globally, 10 million people were suffered from TB and India was reported as the highest TB burden country in the world with an estimated incidence of 26 lakhs (the World Health Organization [WHO] 2020) cases. TB meningitis (TBM) is the common clinical manifestation of central nervous system TB (CNS TB) and is often fatal with long-term neurological consequences. The incidence of TBM is mainly influenced by the overall burden of TB, human immunodeficiency virus (HIV) prevalence, and age criteria.2 3 TBM is an inflammatory meningitis syndrome with nonspecific clinical presentation, and paucibacillary cerebrospinal fluid (CSF) makes the diagnosis extremely difficult. Definitive diagnosis relies on a combination of clinical, radiological, and laboratory findings.4 Early diagnosis and appropriate treatment are important for the proper management of the disease.5 The laboratory diagnostic techniques include observation of acid-fast bacilli (AFB) in CSF smear or isolation of M. tuberculosis in culture. Smear microscopy has a high detection threshold limit and less sensitivity ranging from 0 to 40%.6 7 The liquid culture method, that is, mycobacteria growth indicator tube 960 (MGIT 960) has a lesser detection threshold limit, high sensitivity ranging from 30 to 60% compared with the solid culture method, but results obtain only after weeks of incubation which is too slow to aid in the clinical decision.8 Polymerase chain reaction (PCR)-based assays developed in recent years has been reported with 56% sensitivity and 90% specificity for TBM diagnosis.7 9 The GeneXpert is an automated, real-time PCR that simultaneously detects MTB and resistance to rifampicin (RIF) within 2 hours.10 11 The detection threshold of GeneXpert is 100 to 130 CFU/mL and was found to be useful in detecting both smear-positive and -negative pulmonary TB (PTB) cases.12 13 14 This assay was endorsed by WHO in 2010 and strongly recommended as the preferred initial test for TBM diagnosis.15 GeneXpert sensitivity for EPTB was shown to range from 50 to 80%.16 17 Previous studies have evaluated the performance of this test using a range of CSF volumes combined with and without centrifugation.6 7 8 9 10 18 19 However, studies with a larger population of HIV-positive and -negative cases are needed to confirm the utility of this test in early TBM diagnosis.
Therefore, the present study was aimed to assess the efficacy of GeneXpert for TBM diagnosis using approximately 1 mL of uncentrifuged CSF in volume constraint situations and to determine its usefulness in combination with culture, that is, MGIT 960 in the routine clinical investigation practice.
Materials and Methods
Study Population and Ethics Statement
A prospective study was conducted between 2017 and 2018, in the department of Neuromicrobiology, National Institute of Mental Health and Neurosciences (NIMHANS), a tertiary neurocare center at Bengaluru in India. A total of 235 patients with characteristic signs and symptoms, CSF findings, and impaired neurological function were enrolled as clinically suspected cases of TBM and other neuroinfections as non-TBM. All the patients history and clinical details were obtained from the case records. The diagnosis of the TBM group was made based on the criteria specified in the uniform case definitions described by Marais et al.20 The study was approved by the Institute's Ethics Committee and the written informed consent forms were sought from all the patients.
Sample Collection
A total of 4- to 5-mL CSF was received in our laboratory and based on the patient's clinical details and history; relevant investigations were done for all the patients. The leftover volume of 1.5 to 2 mL was used for AFB culture and the GeneXpert test.
Mycobacterial Investigations
Acid-Fast Bacilli Culture
AFB culture was performed by the liquid culture method in an automated MGIT system with all the safety precautions. Briefly, approximately 500 µL of each CSF volume was inoculated into MGIT tube containing 7-mL Middlebrook 7H9 broth and 0.8 mL of lyophilized MGIT PANTA (containing polymyxin B, azlocillin, nalidixic acid, trimethoprim, and amphotericin B) which was reconstituted with MGIT growth supplement OADC (oleic acid, albumin, and dextrose and catalase). The tubes flagged positive by the instrument were observed for the presence of mycobacteria by smear microscopy and further confirmed by immunochromatographic MPT64 card test (Standard Diagnostic Ltd., Korea).3 4
GeneXpert Assay
The GeneXpert MTB/RIF assay (Cepheid, United States) was performed according to the manufacturer's instructions. In brief, 1 mL CSF of each patient was mixed with 2 mL of sample reagent and incubated for 15 minutes with intermittent shaking. After incubation, 2 mL of the mixture was added into the Xpert cartridge using a sterile pasture pipette and the cartridge was loaded in the instrument. The results were displayed automatically within 2 hours. The reports were given as either “MTB detected” with sensitivity to rifampicin or “MTB not detected.” The cycle threshold (Ct) values will be recorded for each sample with positive test results to assess bacillary load semiquantitatively.7
Statistical Analysis
The statistical analysis was done using Graphpad Prism version 6. The performance of GeneXpert and culture was evaluated in the form of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with a 95% confidence interval (CI). CSF parameters in TBM were compared statistically with that of non-TBM cases. First, normal distribution of data was assessed by Shapiro–Wilk test; S denotes skewness and K denotes kurtosis. Parametric “t”-test or Mann–Whitney U-test (nonparametric) was applied based on the normality distribution to calculate the p-value for assessing the significant differences between the TBM group and control group. A p-value of <0.05 was used to interpret the level of significance.
Result
Patient Details and Clinical and Laboratory Findings
As shown in Fig. 1, a total of 235 cases were included in the study of which 125 were clinically diagnosed as TBM and 110 as non-TBM (controls). Non-TBM group comprised of infectious diseases, namely, cryptococcosis (n = 22), pyogenic meningitis (n = 12), toxoplasmosis (n = 3), brucellosis (n = 1), viral meningoencephalitis (n = 10), Herpes simplex virus (n = 3), measles (n = 1), and noninfectious disorders, like epilepsy, migraine, cerebrovascular disease, polyneuropathy, movement disorders (n = 52), neurodegenerative diseases like multiple sclerosis (n = 3), and carcinomatous meningitis (n = 3).
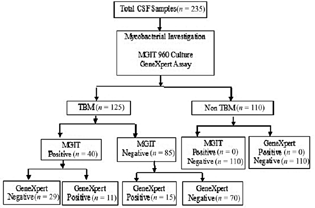
-
Fig. 1 Summary flow chart showing patient recruitment, diagnostic test performed, and test results. CSF, cerebrospinal fluid; MGIT, mycobacteria growth indicator tube; TBM, tuberculous meningitis.
Fig. 1 Summary flow chart showing patient recruitment, diagnostic test performed, and test results. CSF, cerebrospinal fluid; MGIT, mycobacteria growth indicator tube; TBM, tuberculous meningitis.
The majority of the patients included in this study were from Karnataka (77%) and others from neighboring states which included 7.6% from Andhra Pradesh, 6.8% from Tamil Nadu, 6.3% from West Bengal, 1.2% from Bihar, and 0.4% each from Kerala and Jharkhand. Out of 235 cases, 145 (61.70%) were males and 90 (38.29%) were females and the age range was 3 to 80 years (Table 1). The commonly observed signs and symptoms in TBM patients were fever (80%), headache (75.2%), vomiting (55.2%), altered sensorium (48%), loss of appetite (11.2%), and neck stiffness (34.4%). Other unusual clinical findings seen were seizure (12.8%), convulsion (0.8%), meningeal enhancement (10.4%), diplopia (4.8%), photophobia (5.6%), etc. In some patients, a computed tomography (CT) scan showed hydrocephalus (4%), basal exudates (11%), and infarction (0.8%). Among 125 TBM cases, 4 were also diagnosed as miliary TB with TBM.
|
Characteristics |
TBM (n = 125) |
Non-TBM (n = 110) |
|---|---|---|
|
Age (y) Mean ± SD |
36.24 ± 14.81 |
39.27 ± 17.20 |
|
Sex, male/female (%) |
75/50 (60/40) |
70/40 (64/36) |
|
HIV status (P/N/U) |
13/30/82 |
43/37/30 |
|
Evidence of extra CNS-TB, (Yes/No/U) |
4/112/9 |
0/110/0 |
|
Duration of illness (>6 days/< 6 days/U) |
39/46/40 |
90/18/2 |
|
History of contact (Yes/No/U) |
15/101/9 |
0/110/0 |
Abbreviations: N, negative; P, positive; SD, standard deviation; TBM, tuberculous meningitis; U, unknown.
In the TBM group, cytological examination showed characteristic CSF findings like lymphocytic predominant pleocytosis, elevated protein, and low glucose concentration which were found to be statistically significant (p < 0.0001) as compared with the non-TBM (Table 2).
|
CSF parameters Median (IQR) |
TBM (n = 125) |
Non-TBM (n = 110) |
p-Value |
|---|---|---|---|
|
Lymphocytes (cells/µL) |
60 (16–156) |
9(0–35) |
<0.0001 |
|
Protein (g/L) |
185 (102–270) |
88 (51–157) |
<0.0001 |
|
CSF glucose (nmol/L) |
35 (23–54) |
53 (33–73) |
<0.0001 |
|
CSF/serum glucose ratio |
0.3 (0.2–0.4) |
0.3 (0.1–0.5) |
– |
Abbreviations: CSF, cerebrospinal fluid; IQR, interquartile range; TBM, tuberculous meningitis.
Performance of GeneXpert Assay and Culture
As mentioned in the previous section, uniform case definition criteria (Marais and Thwaits, 2010) was followed to ensure the diagnostic certainty of the highest possible standard and to allocate suspected cases of TBM accordingly into the definite, probable, and non-TBM group. Of the total 125 TBM cases, smear microscopy was performed for only 14 cases based on the CSF algorithm followed in the laboratory (chronic meningitis workup, high clinical suspicion, etc.) and staining was done by standard Ziehl–Neelsen method. Of these 14 cases, 3 were detected positive (two probable and one definite) and 11 were identified as negative (seven probable and four definite). Out of 125 TBM cases, 40 (32%) were presented with signs and symptoms, CSF changes (Table 3), cerebral imaging findings suggestive of meningitis, and MTB were detected in CSF by culture, hence categorized as definite TBM.
|
Sr. no. |
Age (y) |
Sex |
CC |
Poly |
Lympho |
Deg |
Protein |
Glucose |
GeneXpert |
RIF S |
|---|---|---|---|---|---|---|---|---|---|---|
|
Case 1 |
23 |
F |
260 |
26 |
234 |
0 |
250 |
11 |
MTB detected very low |
I |
|
Case 2 |
17 |
F |
102 |
0 |
100 |
2 |
887 |
19 |
MTB detected very low |
S |
|
Case 3 |
28 |
F |
120 |
36 |
78 |
6 |
211 |
51 |
MTB detected very low |
R |
|
Case 4 |
2 |
M |
212 |
22 |
186 |
4 |
370 |
50 |
MTB detected very low |
S |
|
Case 5 |
42 |
M |
1,200 |
900 |
300 |
0 |
181 |
33 |
MTB detected very low |
S |
|
Case 6 |
42 |
M |
70 |
20 |
50 |
0 |
490 |
26 |
MTB detected very low |
S |
|
Case 7 |
63 |
M |
190 |
114 |
76 |
0 |
316 |
15 |
MTB detected low |
S |
|
Case 8 |
19 |
F |
180 |
126 |
54 |
0 |
135 |
26 |
MTB detected very low |
S |
|
Case 9 |
27 |
M |
162 |
33 |
1 |
0 |
157 |
14 |
MTB detected very low |
S |
|
Case 10 |
31 |
M |
240 |
0 |
216 |
0 |
149 |
18 |
MTB detected very low |
S |
|
Case 11 |
11 |
F |
50 |
10 |
40 |
0 |
157 |
14 |
MTB detected very low |
S |
Abbreviations: CC, CSF cell count; CSF, cerebrospinal fluid; Deg, degenerated; F, female; I, indeterminate; Lympho, lymphocytes; M, male; MTB, Mycobacterium tuberculosis; Poly, polymorphs; R, resistant; RIF S, rifampicin sensitivity; S, sensitive.
The remaining 85 (68%) cases showed, clinically, CSF and cerebral imaging criteria indicative of meningitis (diagnostic score of >10–12) without an alternative diagnosis.20 All were identified negative by available diagnostic methods performed in our laboratory which includes smear microscopy or culture. Also, none of these had evidence of AFB in the context of histological changes consistent with TB in the brain or spinal cord together with suggestive signs or symptoms and CSF changes (Table 4) or visible meningitis (on autopsy) and therefore designated as probable TBM cases.
|
Sr. no. |
Age (y) |
Sex |
CC |
Poly |
Lympho |
Deg |
Protein |
Glucose |
GeneXpert |
RIF S |
|---|---|---|---|---|---|---|---|---|---|---|
|
Case 1 |
43 |
F |
24 |
13 |
11 |
0 |
121 |
49 |
MTB detected very low |
S |
|
Case 2 |
36 |
M |
16 |
0 |
16 |
0 |
163 |
33 |
MTB detected very low |
S |
|
Case 3 |
27 |
M |
162 |
32 |
130 |
0 |
157 |
14 |
MTB detected very low |
S |
|
Case 4 |
33 |
M |
0 |
0 |
0 |
0 |
104 |
29 |
MTB detected very low |
S |
|
Case 5 |
51 |
M |
154 |
3 |
146 |
5 |
252 |
35 |
MTB detected very low |
S |
|
Case 6 |
31 |
M |
770 |
308 |
462 |
0 |
249 |
28 |
MTB detected very low |
S |
|
Case 7 |
24 |
F |
260 |
0 |
26 |
234 |
250 |
11 |
MTB detected very low |
I |
|
Case 8 |
19 |
F |
180 |
126 |
54 |
0 |
135 |
26 |
MTB detected very low |
S |
|
Case 9 |
35 |
F |
29 |
0 |
19 |
10 |
190 |
3 |
MTB detected very low |
S |
|
Case 10 |
58 |
M |
240 |
120 |
108 |
12 |
465 |
32 |
MTB detected low |
S |
|
Case 11 |
38 |
F |
280 |
0 |
6 |
274 |
220 |
17 |
MTB detected very low |
S |
|
Case 12 |
49 |
M |
8 |
0 |
4 |
4 |
269 |
31 |
MTB detected very low |
S |
|
Case 13 |
39 |
F |
220 |
88 |
132 |
0 |
830 |
4 |
MTB detected very low |
S |
|
Case 14 |
42 |
M |
430 |
43 |
387 |
0 |
199 |
25 |
MTB detected very low |
S |
|
Case 15 |
35 |
M |
110 |
33 |
77 |
0 |
347 |
47 |
MTB detected very low |
S |
Abbreviations: CC, CSF cell count; CSF, cerebrospinal fluid; Deg, degenerated; F, female; I, indeterminate; Lympho, lymphocytes; M, male; MTB, Mycobacterium tuberculosis; Poly, polymorphs; R, resistant; RIF S, rifampicin sensitivity; S, sensitive.
All non-TBM cases were detected M. tuberculosis negative by both the methods. Among 40 definite TBM cases, 11 (28%) were detected positive by GeneXpert of which 9 (81%) were RIF sensitive and 1 (19%) each gave resistant and indeterminate results (Table 2). Out of 85 probable TBM cases, 15 (18%) were detected positive by GeneXpert of which, 14 (93%) were RIF sensitive and 1 (7%) was detected with indeterminate resistance (Table 3). In total 26 (20%) GeneXpert positive cases, 2 (8%) were detected with “low” (Ct; 22–28 cycles) and 24 (92%) with “very low” (Ct; >28 cycles) bacillary load. None of the CSF samples was detected with a “high” or “medium” bacillary load. Machine error was detected in 5 (2%) among 235 CSF samples. GeneXpert Ct values of all the analyzed CSF samples were matching with the semiquantitative bacillary load. As shown in Table 5, the GeneXpert result was evaluated comparatively and in combination with culture as the standard reference method. Samples with machine errors were not included in the analysis. In our study, culture was found to be more sensitive 32% (95% CI: 23.94–40.93%) than GeneXpert. The specificity was given 100% (95% CI: 96.70–100%) by both methods. The sensitivity and specificity of GeneXpert relative to culture was 27% (95% CI: 14.60–43.89%) and 100% (95% CI: 96.70–100%), respectively.
|
Test |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|
|
Culture |
32% (40/125; 23–40%) |
100% (110/110; 96–100%) |
100% |
56.41% (53–59%) |
|
GeneXpert V/S culture |
27% (11/40; 14–43%) |
100% (110/110; 96.70–100%) |
100% |
79.14% (75–82%) |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Discussion
TBM is a medical emergency and is predominantly seen in developing countries like India. Confirmatory diagnosis in the early stages of the infection is very important to effectively control the disease. The problems associated with the currently available conventional and molecular tests demands researchers to look for more rapid and sensitive techniques that could be helpful in the early diagnosis of the disease. GeneXpert assay is now available worldwide and is also currently in use for national TB control programs.21 The study primarily aimed at economizing the CSF volume for TBM diagnosis by GeneXpert in a sample constraint setting. Further, its efficacy in early TBM diagnosis is combined with culture in routine clinical practice. The strength chiefly focused on a large pool of culture-confirmed and probable TBM cases wherein the later, low CSF volume could be relied on as a diagnostic quantity to evaluate the test utility. In the present study, male predominance was observed in the TBM population similar to other studies.7 22 23 The median age among TBM cases for males and females was 35 and 38 years, respectively, revealing the economically productive age group being more vulnerable to TBM.3 5 There was no statistical difference found between the two groups concerning age (p = 0.08) and gender (p = 0.56).5 The signs and symptoms observed in this study were complementary to another study on TBM patients.7 In biochemical parameters, derangement in glucose and protein concentrations was found.6 CSF pleiocytosis with a lymphocytic predominance was observed and the median value of the cell count was 60 cells/mm3.4 The number of HIV-negative cases was more than HIV-positive cases in the TBM population, and this is in agreement with the study population in another part of India.7 This could be due to a lack of information on the HIV status of the patients because for many cases with the infectious disease, the HIV test was not done and some of them were discharged before HIV testing. Previous studies have assessed the performance of GeneXpert for TBM diagnosis with a range of CSF volume and also reported its efficiency using a centrifuged deposit of high CSF volume.5 8 Nhu et al found 59% sensitivity using a medium volume of CSF (2.1–5 mL) and 61% for larger CSF volume (>5 mL).8 A study by Bahr et al also revealed that centrifugation of a large volume of CSF (4–10 mL) before Xpert testing improved the detection rate in TBM reporting 72% sensitivity as compared with 28% using 2 mL uncentrifuged CSF. The later sensitivity is comparable with our study data, where 1 mL uncentrifuged CSF has shown 27% sensitivity and this could be due to more culture-confirmed cases.5 Thus, it is suggestive of considering 1 mL CSF as a representative volume in routine clinical practice because CSF withdrawal is done by an invasive procedure known as lumbar puncture which sometimes can cause serious complications like local skin infection, radicular pain, bleeding, or cerebral herniation.20 So acquiring high CSF volume is difficult not only in children but also in adults with severe neurological disorders. Also diagnosing such a paucibacillary disease that mimics other neuroinfections with a combination of tests can give more confirmatory results than with a single diagnostic test. Hence, 1 mL CSF can be recommended for TBM diagnosis in routine clinical practice against the volume constraint of CSF specimen combined with conventional culture methods.
The present study revealed that culture “the imperfect gold standard” is more sensitive (32%) than GeneXpert (27%). On the contrary, previously, a study reported that in clinically suspected pediatric TBM cases, the assay was able to rapidly confirm the diagnosis of TBM with higher sensitivity (38%) as compared with culture (14%). However, the amount of CSF volume used in their study was relatively more (2 mL) as compared with our study.9 The Sensitivity of GeneXpert found in our study is comparable to a study by Rufai et al in which the test was done using approximately similar CSF volume but the number of TBM cases was more and the study did not include CSF from any non-TBM cases.3 As stated earlier, GeneXpert is a good rule in testing HIV-infected TBM population because of the higher bacterial load in their CSF compared with HIV uninfected.10 In our study, of total TBM cases, the overlap of both GeneXpert positive and culture positive was only 8.8% (11/125). All 3 of 11 HIV coinfected TBM cases were positive by both these methods. Information was not available for the remaining eight cases. About 29 (72.5%) of total culture-positive cases were not detected by GeneXpert and the reason could be due to not enough bacteria to reach the detection threshold limit. Detection is possible only when the required threshold is reached.5 Out of 85 probable TBM cases, 15 (17%) were detected positive by GeneXpert despite a higher analytical detection threshold compared with culture; this might be because of a higher volume of CSF used for the GeneXpert (1,000 µL) than MGIT 960 (500 µL).11 In addition, PCR-positive results in previously treated patients in the absence of culturable bacteria can be due to old DNA or the presence of active disease which is a diagnostic dilemma.12 Two of our probable TBM cases who were HIV negative but detected as GeneXpert positive had a past history of PTB. Both were on antimicrobial treatment more than 10 years back and finally completely recovered. But presently, they were having clinical symptoms suggestive of TBM. GeneXpert positive results in these cases could be true positive, as it was shown earlier that the duration of ≤2 years of previous treatment identified 66% of patients with false-positive results.13 Among 13 probable TBM cases (9.4%) that were detected positive by GeneXpert, HIV status was known only in eight cases of which seven were HIV negative, one was positive and none of them had a past history of TB. Availability of GeneXpert ultra, the modified version of the GeneXpert having a limit of detection of 10 to 100 CFU/mL have increased the MTB detection rate in CSF. Studies have demonstrated higher sensitivity of GeneXpert ultra in HIV infected and uninfected TBM cases using uncentrifuged CSF compared with GeneXpert and MGIT culture.24
Limitations
This study has some limitations that we could not find clinical details for all the patients, and drug susceptibility testing was not performed to confirm the rifampicin sensitivity pattern for culture and GeneXpert positive cases.
Conclusion
In conclusion, the GeneXpert test is highly user friendly which does not require expert handling. Although the test performed well in high CSF volume, CSF sample should be used in priority for GeneXpert in sample volume constraint, as sensitivity depends on the volume of the sample subjected to the test. In our study, in addition to culture-confirmed cases, this assay could detect a considerable number of clinically confirmed culture-negative TBM cases which indicates that it can be a rapid, supplementary test that aids in the early detection of culture-negative cases. Also, the test can be useful where a culture facility is not available.
Acknowledgment
The authors are thankful to the organization, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India, for permitting to carry out the study.
Conflict of Interest
None declared.
Funding This work was supported by National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India. No external funding for this study was reported.
References
- Xpert MTB/Rif for the diagnosis of extrapulmonary tuberculosis–an experience from a tertiary care centre in South India. Trop Med Int Health. 2016;21(3):385-392.
- [Google Scholar]
- Pathogenesis, diagnosis, treatment, and outcome aspects of cerebral tuberculosis. Med Sci Monit. 2004;10(9):RA215-RA229.
- [Google Scholar]
- Pathogenesis and immune response in tuberculous meningitis. Malays J Med Sci. 2014;21(1):4-10.
- [Google Scholar]
- Utility of the Xpert MTB/RIF assay for diagnosis of tuberculous meningitis. PLoS Med. 2013;10(10):e1001537.
- [Google Scholar]
- GeneXpert MTB/Rif to Diagnose Tuberculous Meningitis: Perhaps the First Test but not the Last. Clin Infect Dis. 2016;62(9):1133-1135.
- [Google Scholar]
- Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2015;78(2):47-55.
- [Google Scholar]
- Diagnostic usefulness of Xpert MTB/RIF assay for detection of tuberculous meningitis using cerebrospinal fluid. J Infect. 2017;75(2):125-131.
- [Google Scholar]
- Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat. 2011;2011:798764.
- [Google Scholar]
- Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis. 2018;18(1):68-75.
- [Google Scholar]
- Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48(7):2495-2501.
- [Google Scholar]
- Xpert MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067-1082.
- [Google Scholar]
- Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229-237.
- [Google Scholar]
- Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015.
- [Google Scholar]
- Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis . J Clin Microbiol. 2014;52(6):1846-1852.
- [Google Scholar]
- Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44(2):435-446.
- [Google Scholar]
- Performance of Xpert MTB/RIF on ascitic fluid samples for detection of abdominal tuberculosis. J Lab Physicians. 2017;9(1):47-52.
- [Google Scholar]
- Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49(4):1202-1205.
- [Google Scholar]
- Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52(1):226-233.
- [Google Scholar]
- Improved diagnostic sensitivity for tuberculous meningitis with Xpert MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis. 2015;19(10):1209-1215.
- [Google Scholar]
- Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803-812.
- [Google Scholar]
- Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children. Geneva, Switzerland: World Health Organization; 2013.
- [Google Scholar]
- Diagnosed tuberculous meningitis using cerebrospinal fluid polymerase chain reaction in patients hospitalized with the diagnosis of meningitis in referral hospitals in Isfahan. J Res Med Sci. 2015;20(3):224-227.
- [Google Scholar]
- Diagnosis of tuberculous meningitis: Current scenario from a Tertiary Neurocare Centre in India. Indian J Tuberc. 2017;64(3):183-188.
- [Google Scholar]
- Xpert MTB/RIF Ultra for the detection of Mycobacterium tuberculosis in cerebrospinal fluid. J Clin Microbiol. 2019;57(6):e00249-19.
- [Google Scholar]







