Translate this page into:
Fulminant anti-N-methyl-D-aspartate receptor autoimmune encephalitis presenting as extra-limbic cortical encephalitis
*Corresponding author: Ayush Agarwal, Department of Neurology, All India Institute of Medical Sciences, New Delhi, India. ayushthetaurian@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mustafa F, Garg D, Agarwal A, Garg A, Gupta P, Srivastava AK. Fulminant anti-N-methyl-D-aspartate receptor autoimmune encephalitis presenting as extra-limbic cortical encephalitis. J Neurosci Rural Pract. 2025;16:137-8. doi: 10.25259/JNRP_442_2024
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an autoimmune neurologic disorder characterized by five sequential stages of clinical presentation: a prodromal stage, psychosis or seizure, unresponsiveness or catatonia, hyperkinetic movement disorders, and gradual recovery.[1] The majority of patients present with a subacute onset of psychiatric symptoms, seizures, and movement disorders, followed by dysautonomia and altered sensorium within three weeks of symptom presentation, and have limbic encephalitis on neuroimaging.[2]
An 18-year-old female presented with a headache for eight days, behavioral abnormalities (agitation, irritability, and irrelevant talk) for seven days, and a low-grade fever for five days. In addition, she was noticed to have occasional abnormal posturing involving bilateral upper and lower limbs, occurring intermittently and lasting for 30–60 s, without loss of consciousness. She lost consciousness two days before her presentation to us with worsening of the previously observed posturing episodes. On examination, the Glasgow coma scale score was recorded as E4V2M5, with normal-sized, reactive pupils, and no signs of meningeal irritation. Magnetic resonance imaging (MRI) brain revealed the presence of T2 hyperintensities in the bilateral temporo-occipital cortex (right > left), left hippocampus, and bilateral thalamic (right > left) involvement [Figure 1]. All other routine investigations including viral markers for human immunodeficiency virus and hepatitis B and C were normal. Cerebrospinal fluid (CSF) analysis showed 40 cells (90% polymorphs and 10% lymphocytes), sugar: 79 mg/dL (blood sugar: 112 mg/dL), and protein: 61 mg/dL. Remaining CSF infective workup for neurotropic infections including Gram stain, bacterial and fungal culture, BioFire panel, Ziehl-Neelsen stain, nucleic acid amplification test for tuberculosis (Gene Xpert), India Ink, KOH smear, and cryptococcal antigen were negative. She tested negative for aquaporin-4 and myelin oligodendrocyte glycoprotein (MOG) antibodies in both serum and CSF (fixed cell-based assay). Her paired serum and CSF sample was also tested for autoimmune encephalitis (NMDAR, AMPA1, AMPA2, LGI1, CASPR2, GABA B) panel and her CSF tested positive for anti-NMDAR antibodies. Continuous electroencephalogram monitoring revealed generalized delta slowing with intermittent embedded spike-wave discharges. She was started on optimum doses of intravenous anti-seizure medications, midazolam, and ketamine infusions. She was concomitantly initiated on intravenous methylprednisolone 1 g/daily for five days in conjunction with intravenous immunoglobulin infusion (2 g/kg body weight over five days). Ultrasound of her pelvis was normal. However, she exhibited episodes of severe autonomic dysfunction manifesting as tachycardia-hypertension or bradycardia-hypotension. She succumbed to a sudden cardiac arrest on day 8 of her hospital stay.
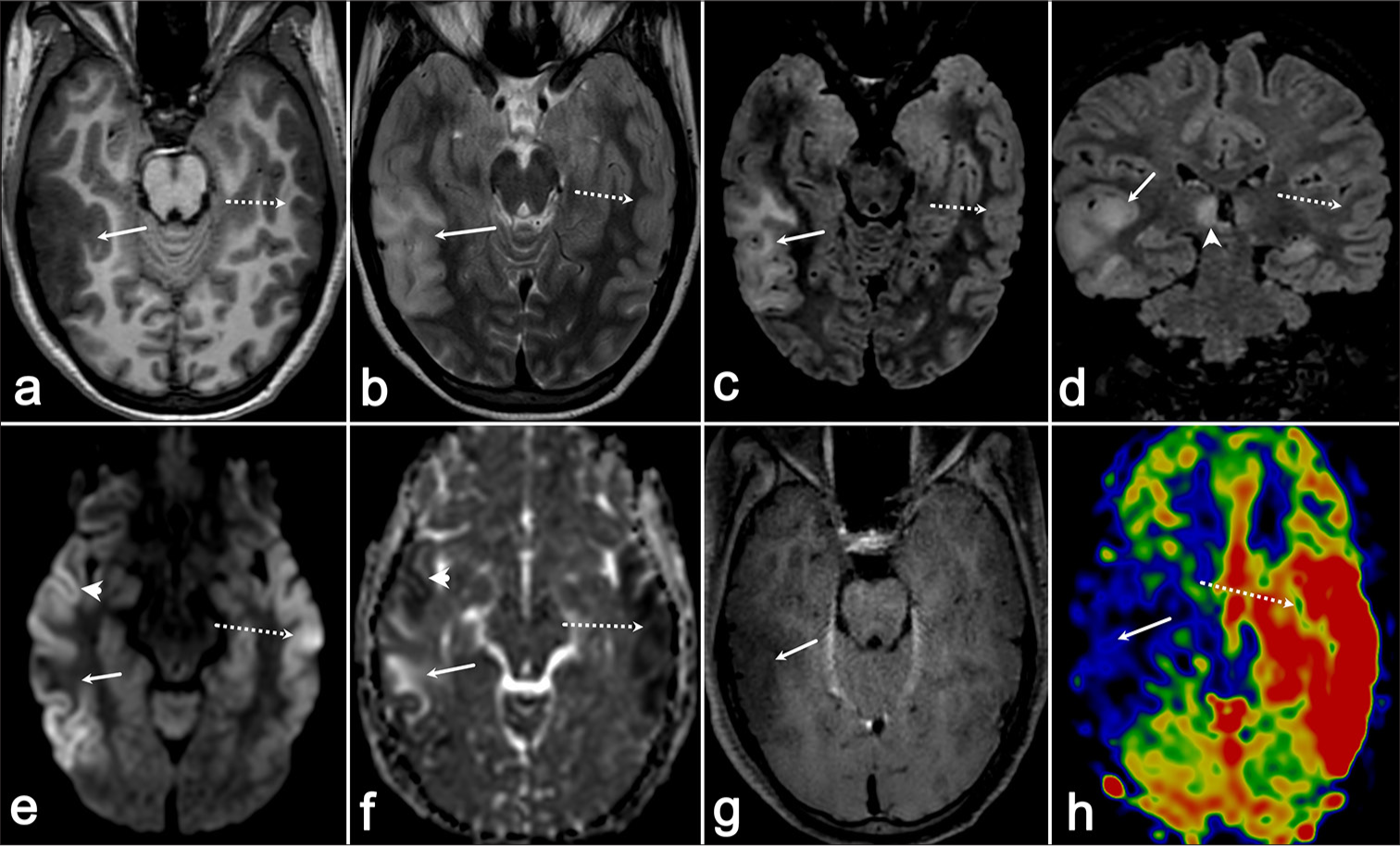
- (a) Axial T1-weighted imaging (T1-WI) demonstrates a hypointense area in the right temporo-occipital region (arrow), which appears (b) hyperintense on axial T2-weighted imaging (T2-WI) (arrow) and (c and d) fluid-attenuated inversion recovery (FLAIR) sequences (arrows). (e) Diffusion-weighted imaging (arrowhead) shows diffusion restriction in the anterior and posterior aspects of this abnormality, which corresponds to (f) hypointensity on the apparent diffusion coefficient map (arrowhead), whereas (e and f) the central portion exhibits facilitated diffusion (arrows). (g) No contrast enhancement is observed on post-gadolinium images, and (h) the arterial spin labeling (ASL) perfusion map reveals reduced perfusion in this region (arrow). (e and f) In addition, there is another area of diffusion restriction in the left temporal region (dotted arrow), which shows (h) increased perfusion on ASL (dotted arrow) and (ad) appears isointense on T1-WI and T2-WI/FLAIR sequences (dotted arrow).
The neuroimaging findings in patients with anti-NMDAR encephalitis are characterized by T2/fluid-attenuated inversion recovery (FLAIR) hyperintensity in the limbic regions: medial temporal lobe, frontal subcortical white matter, and periventricular region, with leptomeningeal enhancement.[1] Dubey et al. have presented a case of anti-NMDAR encephalitis with bilateral symmetric thalamic involvement with diffusion restriction.[2] Chen et al. have described atypical findings of unilateral cortical ribboning in NMDA R encephalitis.[3] However, they had not tested the patient for MOG antibodies and prion disease. Our patient fulfilled the definite criteria for anti-NMDAR encephalitis by Graus et al.[4] and exhibited a combination of these findings, which is extremely rare in anti-NMDAR encephalitis. The common MRI findings in patients with persistent seizure activity are cortical and splenial diffusion restriction, increased perfusion, and occasional contrast enhancement.[5] Our patient had no splenial involvement or contrast enhancement, with hypoperfusion on arterial spin labeling (ASL) and only the anterior and posterior portion of her T2/FLAIR lesion showing diffusion restriction. All other potential causes for these findings were ruled out by relevant investigations. Approximately 80% of patients with anti-NMDAR autoimmune encephalitis improve with immunotherapy, although slowly. Animal models have started to reveal the complex pathophysiology of this disease which might be a reason for the non-improvement/death of the remainder. A detailed understanding of this pathophysiology might lead to the discovery of novel biomarkers and treatments.[6]
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Neurocritical care for Anti-NMDA receptor encephalitis. Biomed J. 2020;43:251-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral thalamic changes in anti-NMDAR encephalitis presenting with hemichorea and dystonia and acute transient psychotic disorder. J Neuroimmunol. 2020;347:577329.
- [CrossRef] [PubMed] [Google Scholar]
- Teaching NeuroImage: Atypical unilateral cortical ribboning in anti-NMDA receptor encephalitis. Neurology. 2022;99:1062-3.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging in status epilepticus: Useful scrying board or expensive stopwatch? Epilepsy Curr. 2023;23:162-5.
- [CrossRef] [PubMed] [Google Scholar]
- An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045-57.
- [CrossRef] [PubMed] [Google Scholar]





