Translate this page into:
Factors predicting outcomes of endoscopic endonasal approach in craniopharyngioma patients
*Corresponding author: Raywat Noiphithak, Division of Neurosurgery, Department of Surgery, Faculty of Medicine, Thammasat University Hospital, Thammasat University, Pathumthani, Thailand. raywat_n@tu.ac.th
-
Received: ,
Accepted: ,
How to cite this article: Taweesomboonyat C, Noiphithak R, Nimmannitya P, Sae-Heng S. Factors predicting outcomes of endoscopic endonasal approach in craniopharyngioma patients. J Neurosci Rural Pract. 2024;15:74-80. doi: 10.25259/JNRP_364_2023
Abstract
Objectives:
Endoscopic endonasal approach (EEA) is commonly used for resection of craniopharyngioma (CP). Treatment outcomes of EEA for CP were related to numerous factors; however, they have been evaluated in few studies. The objective of this study is to investigate factors associated with the outcomes of CP following this operation.
Materials and Methods:
The records of patients with CP, who underwent EEA at our institution from January 2014 to June 2022, were retrospectively reviewed. Surgical outcomes, including the extent of resection, visual recovery, and endocrinological outcomes, were reported. Clinical and radiographic factors were analyzed for their associations with treatment outcomes using logistic regression analyzes.
Results:
This study cohort consisted of 28 patients with CP. Gross total resection (GTR) was achieved in 12 patients (43%). Post-operative visual status improved, stabilized, and deteriorated in 89%, 6%, and 6% of the patients, respectively. There were no patients recovered from pre-operative pituitary dysfunctions, while post-operative hypoadrenalism, hypothyroidism, and hypogonadism were found in 9 (36%), 11 (42%), and 4 (22%) patients, respectively. Post-operative permanent diabetic insipidus was found in 13 patients (50%). Greater suprasellar extension of the tumor was associated with a lower rate of GTR (P = 0.011). Diabetes mellitus (DM) was associated with poor visual recovery (P = 0.022). Larger tumor size and Puget grade 2 were associated with postoperative hypoadrenalism (P = 0.01 and 0.023, respectively). In addition, Puget grade 2 was associated with post-operative hypothyroidism (P = 0.017).
Conclusion:
For EEA in CP, the extent of resection could be determined by suprasellar extension of the tumor. DM was a poor predicting factor for visual recovery, while larger tumors and Puget grade 2 had a higher risk of post-operative hypopituitarism.
Keywords
Craniopharyngiomas
Endoscopic endonasal approach
Transsphenoidal surgery
Extent of resection
Visual recovery
Endocrinological outcome
Predicting factors
INTRODUCTION
Craniopharyngioma (CP) accounts for 2–5% of all primary intracranial neoplasms.[1] Surgery remains the primary treatment modality for CPs.[2] The endoscopic endonasal approach (EEA) is currently recommended for midline CPs due to improved gross total resection (GTR), superior endocrinological and visual outcomes, compared to the transcranial approach.[3,4] Despite the higher rate of cerebrospinal fluid (CSF) leakage in the EEA and microscopic transsphenoidal surgery, the transcranial group had a relatively higher rate of visual deterioration, endocrinological disorders, and post-operative seizure.[4]
The outcome of an EEA for CP patients has been recently evaluated in several studies.[5-14] However, only few studies have determined factors associated with the extent of surgical resection, visual recovery, and endocrine outcomes, after an EEA for CPs. This study aimed to examine factors associated with outcomes following EEA for resection of CPs.
MATERIALS AND METHODS
This study was approved by the local institutional review board following the principles of the Declaration of Helsinki. We retrospectively reviewed the medical records of consecutive patients with CPs who underwent EEA for tumor removal at Thammasat University Hospital from January 2014 to June 2022. Participants were included from the operative records of patients who underwent EEA. Pediatric and adult patients were included in this study. Each patient was evaluated by a team consensus of endocrinologist, ophthalmologist, and neurosurgeon. EEA was indicated for most CPs, except for those exhibiting significant lateral extension or intraventricular location. All surgical procedures were performed by RN as the primary surgeon. An intraoperative computed tomography-guided neuronavigation system was used in all operations. Incomplete data and patients with <6 months of follow-up were excluded from the study. Informed consent was not required given the retrospective nature of this study.
Clinical and radiographic characteristics
Data regarding patients’ characteristics, treatment outcomes, complications, and duration of follow-up were extracted from medical records.
The tumor was classified according to the Kassam classification.[15] The degree of hypothalamic involvement was classified according to the Puget classification.[16] Furthermore, the suprasellar extension of the tumor was measured in millimeters by a perpendicular line from the superior-most part of the tumor to the imaginary line, which was drawn from the tuberculum sellae to the dorsum sellae [Figure 1].
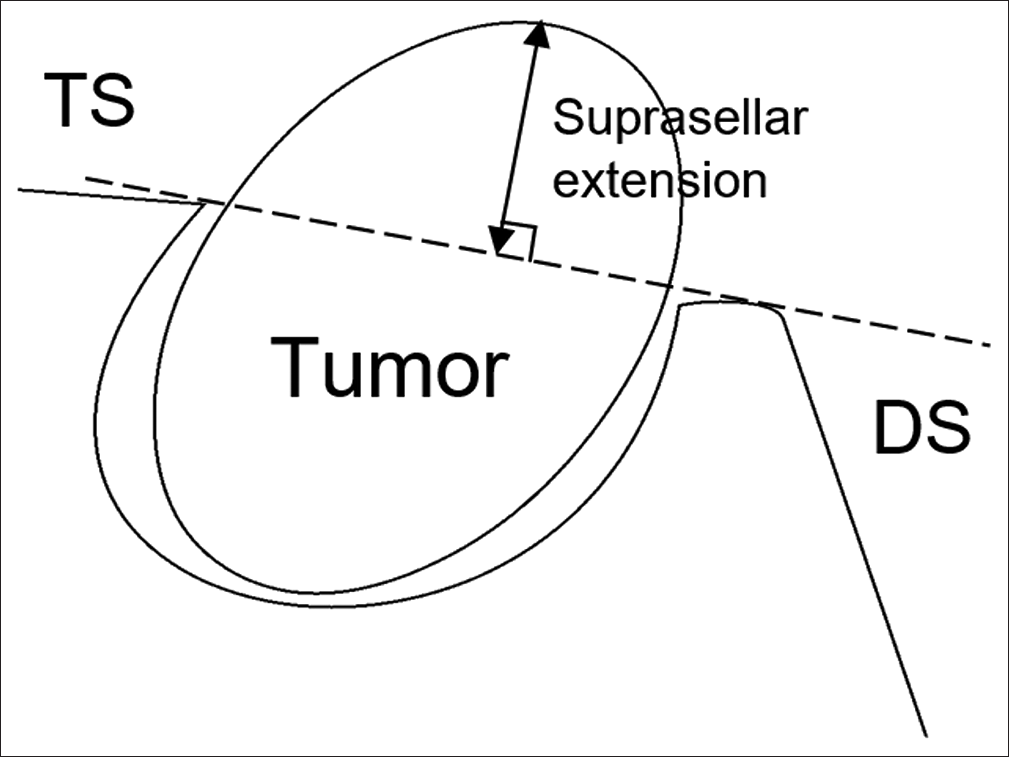
- Measurement of suprasellar extension. An imaginary line was drawn from the TS to the DS, and the suprasellar extension was measured by a perpendicular line from the imaginary line to the superior-most part of the lesion. TS: Tuberculum sellae, DS: Dorsum sellae
The distance between the top of the pituitary gland and the bottom of the optic chiasm was measured on the pre-operative contrast-enhanced T1 weighted MRI in mid-sagittal plane and defined as the chiasm-pituitary corridor (CPC).[8]
The intercarotid distance, Mascarella et al.[17] defined as the smallest distance between the inner walls of the paraclinoid internal carotid artery (ICA) lumen, was measured.
We classified the extent of resection into GTR, near-total resection (NTR), or partial resection (PR), using postoperative contrast-enhanced T1-weighted MRI. GTR was defined as no residual tumor. NTR was defined as residual enhanced tumor or calcification <0.5 cm3, while PR was defined as residual enhanced tumor or calcification ≥0.5 cm3.
Clinical outcomes
Recovery of visual acuity (VA) after treatment was defined as an improvement in logMAR chart of at least 0.1 from pre-operative to the last follow-up. The overall post-operative visual status in each patient was classified as deterioration, stable, or improvement (recovery) compared to the preoperative status. Recovery from cranial nerve (CN) and hormonal deficits were evaluated at the last follow-up evaluation.
Statistical analysis
Continuous variables were reported as mean (standard deviation) whereas categorical variables were reported as number (percentage). Logistic regression analyzes were used to identify factors associated with the extent of resection, recovery of visual impairment, CN, and hypopituitarism, after EEA. Subsequently, the predictors of each outcome were identified using multivariable stepwise backward logistic regression. The final model was determined by the lowest Akaike information criterion values. Statistical analyzes were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) and the Epidemiological Calculator package (R epicalc). P < 0.05 was considered to be statistically significant.
RESULTS
Twenty-eight patients were included in this study. Table 1 showed the baseline characteristics of the patients. Nineteen patients (67.8%) were men with a mean age of 41 ± 19.5 years.
| Characteristic | n(%) |
|---|---|
| Sex, male | 19 (67.8) |
| Age, years; mean (SD) | 41 (19.5) |
| DM | 3 (10.7) |
| Hypertension | 4 (14.2) |
| Body mass index; mean (SD) | 25.5 (5.8) |
| Previous surgery | 4 (14.2) |
| Pre-operative right/left VA deficit | 21 (75)/22 (78.6) |
| Pre-operative right/left VF deficit | 15 (53.6)/14 (50) |
| Visual impairment score; mean (SD) | 49 (33.9) |
| Pre-operative hormonal deficit | |
| Hypoadrenalism | 10 (35.7) |
| Hypothyroidism | 10 (35.7) |
| Hypogonadism | 12 (42.9) |
| DI | 6 (21.4) |
| Pre-operative prolactin level, ng/mL, mean±SD | 28 (16.4) |
Data are presented as n(%); unless indicated otherwise. SD: Standard deviation, DM: Diabetes mellitus, VA: Visual acuity, VF: Visual function, DI: Diabetes insipidus
Three (10.7%) and 4 (14.2%) patients had underlying diabetes mellitus (DM) and hypertension, respectively. The mean body mass index (BMI) was 25.5 ± 5.8. Four (14%) patients underwent CSF diversion before EEA for resections of CPs, and 4 (14.2%) patients underwent previous transcranial approaches for tumor removal. The most common presentation was headache (67.8%), followed by visual impairment (64.2%) and altered consciousness (35.7%).
Pre-operative VA deficit was found in 21 (75%) and 22 (78.6%) of patients in the right and left eyes, respectively. Pre-operative VF deficit was found in 15 (53.6%) and 14 (50%) of patients in the right and left eyes, respectively. The lower incidence of VF deficit resulted from an inability to accurately measure VF in patients with poor VA or those with altered mental status. The mean pre-operative VIS was 49 ± 33.9. CN III and VI deficits were found in 2 (7%) and 1 (4%) patients, respectively. Pre-operative hypoadrenalism, hypothyroidism, and hypogonadism were found in 10 (35.7%), 10 (35.7%), and 12 (42.9%) patients, respectively. Pre-operative diabetes insipidus (DI) was found in 6 (21.4%) patients. The mean pre-operative serum prolactin was 28 ± 16.4 ng/mL.
Radiographic characteristics of the tumor are shown in Table 2. The mean tumor size and volume were 32.1 ± 8.6 mm and 11.8 ± 8 cm3, respectively. The mean suprasellar extension of the tumor was 22.2 ± 9.3 mm. The mean width of the CPC was 6.1 ± 3.2 mm. The inter carotid distance measured at the level of the paraclinoid ICA was evaluated and the mean was 13.7 ± 2.5 mm. Most of the tumors (53.5%) did not have calcification. Eight (28.6%) and five (17.9%) tumors had calcification >10% and <10% of tumor volume, respectively.
| Radiographic characteristic | n(%) |
|---|---|
| Tumor size, mm; mean (SD) | 32.1 (8.6) |
| Tumor volume, cm3; mean (SD) | 11.8 (8) |
| Suprasellar extension of lesion, mm; mean (SD) | 22.2 (9.3) |
| Width of the chiasm-pituitary corridor, mm; mean (SD) | 6.1 (3.2) |
| Intercarotid distance, mm; mean (SD) | 13.7 (2.5) |
| Calcification within the tumor | |
| No | 15 (53.5) |
| <10% of tumor volume | 5 (17.9) |
| >10% of tumor volume | 8 (28.6) |
| Kassam classification | |
| Preinfundibular | 9 (32.1) |
| Transinfundibular | 5 (17.9) |
| Retroinfundibular | 14 (50) |
| Hypothalamic involvement by Puget | |
| Grade 0 | 4 (14.3) |
| Grade 1 | 8 (28.6) |
| Grade 2 | 16 (57.1) |
| Lateralization of tumor beyond internal carotid artery bifurcation | 5 (17.9) |
Data are presented as n(%); unless indicated otherwise. SD: Standard deviation
Pre-operative MRI showed that 50% of the tumors were retro infundibular types, followed by preinfundibular (32.1%) and transinfundibular (17.9%) types, respectively. Sixteen (57.1%) patients had hypothalamic involvement of Puget grade 2. Five (17.9%) tumors had lateral extension beyond ICA bifurcation on one or both sides.
During surgery, the pituitary stalk was transected in 6 (21.4%) patients. In 7 (25%) operations, the pituitary transposition technique was used to increase the surgical exposure. Ten (35.7%) patients received adjuvant treatment, including 1 (3.6%) reoperation, 4 (14.2%) radiation therapy, and 5 (17.9%) combined treatment. The median duration of follow-up was 9.5 months. Some patients were sent to follow-up at their nearby hospital if further treatment was not required.
Treatment outcomes
After EEA for CP resection. GTR was achieved in 12 patients (42.8%), while 8 patients (28.6%) achieved NTR and PR equally. Moreover, 13 (61.9%) and 14 (66.7%) patients had improved VA in their right and left eyes, respectively. All patients with preoperative CN deficits recovered completely. From 10 patients with preoperative hypoadrenalism, 2 (20%) patients recovered from hypoadrenalism after the operation. No patient recovered from pre-operative hypothyroidism or hypogonadism. Two (33.3%) of 6 patients with pre-operative DI recovered from DI after the operation.
Predicting factors of the extent of resection
There were no significant clinical and radiographic characteristics associated with the extent of resection (GTR vs. NTR and PR). The predicting model of the extent of surgical resection consisted of BMI, (odds ratio [OR] 1.2, 95% confidence interval [CI] 0.96–1.51, P = 0.107) pre-operative prolactin level (OR 0.93, 95% CI 0.87–1.01, P = 0.075), and suprasellar extension (OR 1.16, 95% CI 1.01–1.33, P = 0.03). Greater suprasellar extension of the tumor was the only significant factor associated with a higher rate of non-GTR [Table 3]. Four (66.7%) of six patients with suprasellar extension <15 mm achieved GTR from surgery, while only 8 (36.4%) of 22 patients with suprasellar extension ≥15 mm achieved GTR.
| Outcome | Factor | OR (95% CI) | P-value |
|---|---|---|---|
| Extent of resection | BMI | 1.2 (0.96, 1.51) | 0.107 |
| (GTR vs. NTR and PR) | Preoperative prolactin level | 0.93 (0.87, 1.01) | 0.075 |
| Suprasellar extension | 1.16 (1.01, 1.33) | 0.03 | |
| Visual recovery | Age | 1.07 (0.94–1.22) | 0.037 |
| DM | 30 (1.3–693.13) | 0.997 | |
| Hypertension | 14 (0.83–235.08) | 0.997 | |
| Hypoadrenalism | Tumor size | 1.15 (0.92–1.44) | 0.233 |
| Puget grade 2 (vs. grade 0–1) | 2.7 (0.07–111.34) | 0.601 | |
| Hypothyroidism | Tumor size | 1.06 (0.89–1.26) | 0.546 |
| Puget grade 2 (vs. grade 0–1) | 9.85 (0.42–233.6) | 0.157 |
BMI: Body mass index, GTR: Gross total resection, NTR: Near-total resection, PR: Partial resection, OR: Odds ratio, CI: Confidence interval, DM: Diabetes mellitus
Predicting factors of the visual and endocrinological outcomes
For visual outcome, DM was the only factor significantly associated with no recovery (P = 0.022). One of three (33%) patients with underlying DM gained visual recovery after surgery, while 15 of 16 (94%) patients without underlying diabetes gained visual recovery after surgery. The final model of predicting factors of visual recovery consisted of age (OR 1.07, 95% CI 0.94–1.22, P = 0.307), DM (OR 30, 95% CI 1.3–693.13, P = 0.997), and hypertension (OR 14, 95% CI 0.83–235.08, P = 0.997). However, none of these factors were significant [Table 3].
For endocrinological outcomes: Larger tumor size and Puget grade 2 (vs. grade 0-1) were significantly associated with post-operative new hypoadrenalism (P = 0.01 and 0.023, respectively) but only Puget grade 2 was significantly associated with postoperative new hypothyroidism (P = 0.017). Tumor size and Puget grade 2 were identified in the final predicting model of both postoperative hypoadrenalism and hypothyroidism. However, none of these factors were significant [Table 3].
Due to the low incidence of pre-operative CN III–VI deficit and all patients with pre-operative deficit recovering from CN palsy, predicting factors of CN outcomes could not be investigated.
Post-operative complications
There were 9 patients (32%) who had post-operative CSF leakage, of which 2 (7%) had meningitis. All of these patients underwent surgical repair for CSF leakage. Postoperative new hypoadrenalism, hypothyroidism, and hypogonadism were found in 9 (36%), 11 (42%), and 4 (22%) patients, respectively. Post-operative new permanent DI was found in 13 patients (50%). There was no intracranial vessel injury or post-operative bleeding complications [Supplementary Table 1].
DISCUSSION
This study included patients with CP who underwent EEA in a single institution. The characteristics of our cohort in this study were similar to the previous studies. The mean age of the patients in this study was within the range of 30.6–51.1 years, as reported in the literature.[5-7,9-14] In addition, the mean BMI in this study corresponded to the range of 24.6–27.6, as previously reported.[5,9,10,12] For the tumor characteristics, the mean tumor size in this study was 32.1 mm, slightly larger than the range of 25.0–29.0 mm that has been reported in the literature.[6-8,10,12,13] Fomichev et al.[11] reported that tumor diameter larger than 42 mm was associated with a high mortality rate of 5.8%, compared to the rate of 0–0.7% in the literature.[5-10,12] In addition, 16 patients (57%) had hypothalamic involvement of Puget grade 2, which was slightly more than the range of 42–49.3% in the previous studies.[7,17]
Regarding the treatment outcomes, GTR was achieved in 43% of the patients in this study, which was slightly lower than the range of 38–91% range of the GTR rate in the previous studies.[5-7,9-12] The low rate of GTR in this study might be related to our early experience in EEA for resection of CPs. The study by Ceylan et al.[6] and Park et al.[10] reported a rate of 49% and 20% GTR in their initial 33 and 20 patients who underwent EEA. These studies also found that surgical experience was significantly associated with GTR outcome.[6,10] In addition, larger tumor size and higher Puget grade in our cohort, compared to the previous studies, could explain a lower GTR rate in our study. Greater suprasellar extension of the tumor was significantly associated with a lower rate of GTR. In addition, suprasellar extension could be a quantitative measurement for hypothalamic involvement due to the strong correlation between these two variables. As a result, tumors with greater suprasellar extension, especially ≥15 mm, could preclude GTR [Figure 2]. The previous studies found that lack of surgical experience,[6,10] previous surgical treatment,[6,7,9,12] tumor volume ≥10 cm3,[5] and suprasellar location (vs. infrasellar-sellar location)[6] were significantly associated with a non-GTR after EEA. The study by Omay et al.[8] and Kim et al.[7] found that CPC and low-lying chiasm were not significantly associated with the extent of resection.

- Magnetic resonance imaging of example cases showing suprasellar extension of craniopharyngioma as a predictor of extent of resection. (a) A case with an 8-mm suprasellar extension of the tumor (b) achieved gross total resection (c). A case with a 22.3-mm suprasellar extension of the tumor (d) achieved subtotal resection.
Post-operative visual function improved in 89% of our cohort, comparable to 56–86% in the literature.[5-7,9,13] DM was significantly associated with persistent visual deficit. Although CPs directly compress the optic apparatus, the visual function in patients with DM can be worsened due to diabetic-related diseases, such as retinopathy, macular degeneration, ischemic optic neuropathy, or cataracts, resulting in poor recovery. Dho et al.[9] found that previous surgery was associated with a less visual improvement.
The incidence of post-operative new anterior hypopituitarism and DI in this study was not different from the previous literature, ranging from 13% to 47.4%[6,8-10] and 21–66%,[5,6,8-10,12] respectively. The association between pre-operative risk factors and post-operative new hormonal deficit has not been well reported in the previous studies. Here, we found that larger tumor and Puget grade 2 were significantly associated with post-operative new hypopituitarism [Figure 3]. Manipulating of the hypothalamic-pituitary axis during resection of CP with these features can lead to postoperative hypopituitarism. For DI, although Godil et al.[5] found that GTR resulted in a higher rate of DI, we did not found this correlation in our cohort. In contrast, there were 2 of 10 patients (20%) with preoperative hypoadrenalism in this study recovered from this deficiency after the operation. Two of 6 patients (33%) with pre-operative DI could discontinue usage of desmopressin in their follow-up period. Although recovery of pituitary function was not the primary goal of surgical treatment, the previous studies reported improvement of pituitary function in 0–20% of patients with pre-operative deficit.[6,9-11,13]

- Magnetic resonance imaging of example cases showing hypothalamic involvement of craniopharyngioma as a predictor of endocrinological outcomes. (a) A case with a Puget grade 0 (b) underwent total tumor resection with post-operative normal pituitary function (c). A case with a Puget grade 2 (d) underwent subtotal tumor resection with postoperative panhypopituitarism.
The most common complication of EEA for CP in this study was postoperative CSF leakage (32%), which was higher than 6.8–23.4% in previous studies.[5-7,9-11,13,14] In addition, post-operative meningitis occurred in 7% of patients in this study, compared to 2.3–16% in the previous studies.[5,10,11,13] In our cohort, post-operative CSF leakage occurred in 9 of 20 patients who underwent EEA before June 2021 while this complication did not occur in the last 8 patients after June 2021. This indicated that the learning curve of EEA for CP was an important factor in determining the incidence of post-operative CSF leakage. Kshettry et al.[14] reported a 40% rate of post-operative CSF leakage at 40% in their early cohort, which decreased to 4% in their subsequent cohort. In addition, in EEA, the skull base reconstruction technique plays a key role in the prevention of CSF leakage. Several authors suggested the use of vascularized septal flap, especially in pediatric patients, for the reconstruction in EEA for CPs to prevent CSF leaks.[18,19] Zwagerman et al.[20] found that post-operative lumbar drainage significantly reduced the rate of postoperative CSF leakage in patients who underwent EEA with intraoperative high-flow leakage. However, post-operative lumbar drainage had not been routinely performed in our institution, which might have contributed to the increased risk of CSF leakage. Despite this, no intracranial vessel injury or post-operative bleeding occurred in this study.
There were several limitations in this study. First, a single-center retrospective nature of this study might introduce biases. However, all surgical procedures in this study were performed by a single surgeon; thus, eliminating potential variability in surgical capability and treatment strategy. In addition, we utilized the multivariate analysis to ameliorate these limitations. Second, the sample size was not large enough for the generalizability. Third, this cohort included our early experience in EEA, affecting a relatively low rate of GTR and a high rate of postoperative CSF leakage. However, to the best of our knowledge, this is the first study to identify the factors associated with outcomes after EEA for CP resection.
CONCLUSION
For CP patients who underwent EEA, the extent of resection could be quantitatively determined by the suprasellar extension of the tumor. In term of surgical outcomes, DM was a poor predictor of visual recovery, while the larger tumor and Puget grade 2 had a higher risk of post-operative hypopituitarism. These predicting factors can be used to optimize the treatment strategy for CP patients to improve outcomes and minimize post-operative complications.
Ethical approval
This study was approved by the local institutional review board following the principles of the Declaration of Helsinki.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- Craniopharyngiomas. Endocrinol Metab Clin North Am. 2008;37:173-93, ix-x
- [CrossRef] [PubMed] [Google Scholar]
- Update on management of craniopharyngiomas. J Neurooncol. 2022;156:97-108.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of craniopharyngiomas in adult patients: A systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien). 2020;162:1159-77.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012;77:329-41.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term tumor control after endoscopic endonasal resection of craniopharyngiomas: Comparison of gross-total resection versus subtotal resection with radiation therapy. J Neurosurg. 2022;136:1347-55.
- [CrossRef] [PubMed] [Google Scholar]
- An endoscopic endonasal approach to craniopharyngioma via the infrachiasmatic corridor: A single center experience of 84 patients. Acta Neurochir (Wien). 2021;163:2253-68.
- [CrossRef] [PubMed] [Google Scholar]
- Is low-lying optic chiasm an obstacle to an endoscopic endonasal approach for retrochiasmatic craniopharyngiomas? (Korean Society of Endoscopic Neurosurgery-003) World Neurosurg. 2018;114:e306-16.
- [CrossRef] [PubMed] [Google Scholar]
- Is the chiasm-pituitary corridor size important for achieving gross-total resection during endonasal endoscopic resection of craniopharyngiomas? J Neurosurg. 2018;129:642-7.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic endonasal approach for craniopharyngioma: The importance of the relationship between pituitary stalk and tumor. J Neurosurg. 2018;129:611-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcome after extended endoscopic endonasal resection of craniopharyngiomas: Two-institution experience. World Neurosurg. 2017;103:465-74.
- [CrossRef] [PubMed] [Google Scholar]
- Extended transsphenoidal endoscopic endonasal surgery of suprasellar craniopharyngiomas. World Neurosurg. 2016;94:181-7.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome and mid-term prognosis after maximum and radical removal of craniopharyngiomas with the priority to the extended transsphenoidal approach-a single center experience. Clin Neurol Neurosurg. 2014;125:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic endonasal surgery for craniopharyngiomas: Surgical outcome in 64 patients: Clinical article. J Neurosurg. 2013;119:1194-207.
- [CrossRef] [PubMed] [Google Scholar]
- The learning curve in endoscopic endonasal resection of craniopharyngiomas. Neurosurg Focus. 2016;41:E9.
- [CrossRef] [PubMed] [Google Scholar]
- Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: A new classification based on the infundibulum. J Neurosurg. 2008;108:715-28.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric craniopharyngiomas: Classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106:3-12.
- [CrossRef] [PubMed] [Google Scholar]
- Indicators of a reduced intercarotid artery distance in patients undergoing endoscopic transsphenoidal surgery. J Neurol Surg B Skull Base. 2015;76:195-201.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic skull base reconstruction of large dural defects: A systematic review of published evidence. Laryngoscope. 2012;122:452-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23:518-21.
- [CrossRef] [PubMed] [Google Scholar]
- Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg. 2018;131:1172-8.
- [CrossRef] [PubMed] [Google Scholar]
SUPPLEMENTARY TABLE
| Complication | n(%) |
|---|---|
| Cerebrospinal fluid leakage | 9 (32) |
| Meningitis | 2 (7) |
| New hypopituitarism | |
| New hypoadrenalism | 9 (36) |
| New hypothyroidism | 11 (42) |
| New hypogonadism | 4 (22) |
| New permanent diabetes insipidus | 13 (50) |
Data are presented as n(%)







