Translate this page into:
Experimental study of neuropharmacological profile of Euphorbia pulcherrima in mice and rats
Address for correspondence: Dr. Kundan Kr. Singh, Department of Clinical Pharmacology and Therapeutics, B.P. Koirala Institute of Health Sciences, Dharan, Sunsari, Nepal. E-mail: kundansngh@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Euphorbia pulcherrima (EP) belongs to the family: Euphorbiaceae and Genus: Euphorbia. Many species of Euphorbia have been reported as having beneficial properties like anticonvulsive effect, central analgesic properties, antipyretic action, central depressant action and strong sedative effect. However, little study has been done and published on EP.
Aims:
To observe and evaluate various neuropharmacological effects like antinociceptive effect, anticonvulsant effect, motor in-coordination, pentobarbital induced sleeping time and behavioral responses of EP in mice and rats.
Setting and Design:
Quantitative experimental study in mice and rats by various experimental models.
Materials and Methods:
Different experimental models were used to assess the antinociceptive effect (hotplate, tail flick and acetic acid induced writhing test), anticonvulsant effect (Maximal Electroshock Seizure test [MES] and Pentylenetetrazole induced seizures [PTZ]), motor in-coordination effect (Rota rod test), pentobarbital induced sleeping time and behavioral responses of EP in mice and rats after oral administration of EP crude dried extracts in three different doses (250, 500 and 1000 mg/kg).
Statistical Analysis Used:
The significance of difference with respect to control was evaluated using the Mann-Whitney U test. A probability (P-value) level less than 0.05 was considered as significant.
Results:
In MES test model, duration of tonic hind limb extension in mice treated with EP was significantly less as compared to vehicle treated group. EP was most effective in a dose of 1000 mg/kg. There was also significant increase in the latency and decrease in the incidence of convulsions with the use of EP in three different doses in PTZ induced seizure model.
Conclusions:
This study showed EP (crude dried) extracts to possess anticonvulsant properties but no effect on motor co-ordination and anxiety.
Keywords
Anticonvulsant effect
antinociceptive effect
anxiolytic
pentobarbital induced sleeping time
sedative
Introduction
Euphorbia pulcherrima (EP) belongs to the family: Euphorbiaceae and Genus: Euphorbia. (English name: Poinsettia [Christmas star], Nepali name: Lalupate, Hindi name: Lalpatta).[1] It is commonly referred to as poinsettia, flowers of Mexican origin, native to the Pacific coast of the United States, some parts of central and southern Mexico (including the Mexican Pacific coast), and a few localities in Guatemala. In addition, it is also easily found all over Nepal, especially in the hilly region.
Leaves and flowers of the EP plant have been used as an indigenous drug in different conditions like skin disease, to increase the secretion of milk in nursing mothers and as a mild laxative.[1] Many species of Euphorbia have been reported as having beneficial properties like central analgesic properties, antipyretic action, central depressant action and strong sedative effect.[23] Methanolic extracts of Euphorbia calyptrate root causes a significant reduction in general behavioral profile, potentiates Phenobarbital-induced sleeping time and reduces spontaneous motor activity.[4] Hydro-alcoholic extract of Euphorbia neriifolia has anti-anxiety, anti-psychotic, and anticonvulsant action in mice and rats.[5] Lyophilised aqueous extract of Euphorbia hirta has been reported to have sedative and anxiolytic effects along with a strong reduction in the release of prostaglandins I2, E2, and D2.[67] In addition, it has shown to exert an inhibitory effect on platelet aggregation and depress the formation of carrageen induced rat paw oedema.[8] Euphorbia royleana latex has shown analgesic and antipyretic properties in hyperthermic experimental model of rats and rabbits.[9] In a placebo controlled single blind study, Euphorbia fisheriana produced significant antiepileptic effect compared with placebo.[10]
The latex, stems, bracts, and flowers of EP have been chemically studied by extraction with petroleum ether. All the EP extracts have been shown to contain germanicol, p-amyrin, and pseudotaraxasterol and the latex to contain a new sterol, C24H40O, pulcherrol. From the extracts of the stems have been isolated an octaeicosanol and 8-sitosterol.[11] Despite extensive phytochemical research on plants of Euphorbiaceae families, little study has been done and published on EP. Since many of the pharmacological effects of Euphorbiaceae family appear to be related to central nervous system (analgesic, anti-inflammatory and antipyretic properties, behavioral and neuropharmacological effects like anticonvulsant, hypnotic and neuroleptic properties), it was planned to further study the neuropharmacological effects of the EP on various parameters like antinociceptive effect, anticonvulsant effect, motor in-coordination, pentobarbital induced sleeping time and behavioral responses by several experimental models, which implicates its effects on the CNS.
Materials and Methods
Drugs and chemicals
Indomethacin (Indocap, Jagsonpal Pharmaceuticals, India); Phenytoin (M-Toin, Medopharm, India); Sodium valproate (Encorate, Sun Pharmaceuticals, India); Diazepam (Valium, Piramal Healthcare, India); Pentylenetetrazole (Sigma Chemicals, USA); Pentobarbital (Loba Chemie, India); Morphine (Martindale Pharmaceuticals, UK); Acetic acid (Qualigens fine chemicals, India)
Animals
Experiments were performed on adult albino mice (n = 240) weighing 20-30 g and Wistar albino rats (n = 0) weighing 100-200 g. Animals were bred in the laboratory breeding house of the Department of Clinical Pharmacology and Therapeutics, B.P. Koirala Institute of Health Sciences, Dharan, Nepal. The animals were maintained under controlled room temperature (25 ± 2°C) and light and dark (12:12 hr) conditions and were given food pellets and water ad libitum. Animals were fasted overnight before the experiment. Before conducting the experiment, ethical clearance was obtained from the local Ethical committee on Animal Research and ethical guidelines for investigations were followed in accordance with Indian National Science Academy (INSA) guidelines.
Each experiment consisted of five groups of animals, six in each group. Group I (vehicle control, vehicle treated); Group II (standard control), Group III (test drug, Euphorbia pulcherrima 250 mg/kg), Group IV (test drug, Euphorbia pulcherrima 500 mg/kg) and Group V (test drug, Euphorbia pulcherrima 1000 mg/kg).
Drugs preparation
Euphorbia pulcherrima (EP) was identified with the help of available literature and resources. Milky latex of the plant was collected from leaf and stem in petri dish which was allowed to dry at room temperature for 15 days. After 15 days, it was recovered from the petri dish with the help of new scalpel blade and finally grinded to fine powder with mortar and pestle. Vehicle for the study was distilled water (10 ml/kg). EP crude dried latex suspension was made by mixing with distilled water. Also, Indomethacin, Morphine, 0.6% Acetic acid, Pentylenetetrazole, Phenytoin, Valproic acid, Diazepam and Pentobarbitone were dissolved in distilled water.
Test drug, standard control drugs and vehicle was given through oral route with the help of orogastric tube.
The standard control for the analgesic effect was Morphine 5 mg/kg (hot-plate and tail-flick test) and Indomethacin 20 mg/kg (writhing test). Standard controls for anticonvulsant effect were Phenytoin 10 mg/kg (maximal electroshock seizure) and Sodium valproate 300 mg/kg (PTZ induced seizure). Diazepam 5 mg/kg was the standard control for effect on motor co-ordination; Diazepam 3 mg/kg for pentobarbital induced sleeping and Diazepam 1 mg/kg for anxiolytic effect (open-field and passive avoidance tests).
Experimental designs
Antinociceptive effect
Hot plate method
The hot plate test was carried out according to the method described by Eddy and Leimbach.[1213] The thermal noxious stimulus was induced in mice by placing them in a hot-plate (UGO Basile, Italy) maintained at 53°C 10 min prior to the experiment. Drugs were given orally 45 min before placing the mice in hot-plate and the reaction time (hot-plate latency) was recorded.[14] Mice with basal latency of more than 10 seconds were not included in the study.
Tail – flick method
The central antinociceptive effect was determined using the tail flick test.[15] For the tail-flick method, pain was induced by giving infrared light on the tail of the mice (Tail-Flick Unit, UGO Basile, Italy) 5 cm away from the tip of the tail. Reaction time (tail-flick latency) was noted by observing the interval between placing the tail on the infrared light source and the withdrawal of the tail.[13] A maximum radiation exposure period of 30 seconds was taken as the cut off time. Drugs were given orally 45 min prior to the test.
Writhing test
The antinociceptive activity was assessed using writhing test (abdominal constriction test).[16] This assay procedure is considered as very sensitive with minimal noxious stimulus.[17] After 45 min of drug administration, the number of abdominal constrictions (writhings) in mice for a period of 15 min was counted following intraperitoneal injection of 0.6% acetic acid in a dose of 10 ml/kg. Any significant reduction in the number of abdominal constrictions when compared with vehicle-treated animal was considered as antinociceptive response.[18] Standard control for this test was Indomethacin (20 mg/ kg, PO). Antinociception was expressed as the number of abdominal constrictions between distilled water treated control and animals pretreated with test drug or Indomethacin.
Anticonvulsant effect
Maximal Electroshock Seizure (MES) test
The MES pattern was induced in animals by using a convulsiometer (Techno, India) to give an alternating current of 150 mA for 0.2 sec. Forty-five min post dosing, mice were subjected to MES of 150 mA of alternating current from a convulsiometer for 0.2 second through a pair of electrodes attached to each ear.[19] The duration of the tonic hind limb extensor phase, clonic phase and the number of animals protected from convulsions was noted. Phenytoin in doses of 10 mg/kg PO was used as standard control.
Pentylenetetrazole (PTZ) induced seizures
PTZ was used in a dose of 40 mg/kg, intraperitoneally. This is the dose that produces clonic seizures without mortality in all the animals. The test drug was administered 45 min prior to PTZ administration. The latency to first convulsion and the number of mice, which exhibited seizures, were observed immediately after the test drug injection for a period of 30 min.[20]
Motor co–ordination (Rotarod) test
A rotarod tread mill device (Techno, India) was used for the evaluation of motor coordination, which consisted of a horizontal metal rod coated with rubber with 3 cm diameter attached to a motor with the speed adjusted to 16 rotations per min (16 RPM). The rod was 75 cm in length, divided into 6 sections by plastic discs, thereby allowing the simultaneous testing of 6 mice. The rod was at a height of about 50 cm above the table top in order to discourage the animals from jumping off the roller. Cages below the sections serve to restrict the movements of the animals, when they fell from the roller. Experimental animals were subjected to a pretest on the apparatus. Only those animals, which demonstrated their ability to remain on the revolving rod for at least one min, were used for the test. The test compound was administered orally. One hour after the administration of drugs, each mouse was placed on the rotating rod for 60 seconds, at intervals of 30 min for 2 hr. The endurance time for each mouse on the rota-rod was noted.
Pentobarbital induced sleeping time
This test was carried out by sedating rats using Pentobarbitone sodium. Forty- five min after administration of drug or vehicle, all the animals received 40 mg/kg i.p. of Pentobarbitone sodium. Sleeping time was calculated as the interval between the loss and the recovery of the righting reflex.[21]
Anxiolytic effect
Open field test
This is an experimental model for assessment of anxiogenic activity and locomotor activity.[22] In the open field test (OFT), confrontation with the situation induces anxiety behavior in rodents. The OFT apparatus consisted of a wooden box (40 cm ´ 40 cm with 30 cm high walls); with painted black floor, subdivided into nine equal fields by white lines. The experimental room is a sound attenuated dark room. The OFT box, illuminated with a 40 W bulb, placed at a height of about 50 cm, was placed in the experimental room. After 45 min of drug administration, mice were placed individually in a corner square of the box and the ambulation (the number of squares crossed at periphery), total locomotion (total number of squares travelled), activity in the center (number of central squares crossed) and rearing (number of times the animal stands on the rear paws) was recorded for 5 min.[23] Rearing reflects an exploratory tendency[24] of the animal that can be reduced due to a high level of fear. Enhanced peripheral, central and total number squares crossed are taken as increased locomotor activity. In addition, increased rearing, number of inner squares crossed and time spent in them reflects enhanced exploratory activity and reduced fear.[25] All the above parameters are inversely proportional to the level of anxiety. The observation was made on a closed circuit TV.
Passive avoidance response (PAR) test
PAR is extensively used for the screening of drugs affecting learning and memory.[26] The test involves training rodents to avoid punishment (normally an electric shock) by curbing a normal behavior (such as the exploratory behavior). At specified intervals after training, the animals were tested again for retention of such learning.
A study chamber measuring 34 cm × 34 cm × 20 cm was used through the metal floor of which electric shock of 20 mv was delivered on contact. A shock free zone (SFZ) was provided in the centre of the chamber by placing an inverted Petri dish. Mice were initially placed on the shock free zone and they got down on the grid floor where they received an electric shock. A reduction in the natural anxiety on exposure to such a situation was implicated by a decrease in the latency to step down and an increase in the number of step down errors.[27] The parameters recorded during passive avoidance behavior were:
-
Step down latency (duration for which the animal stayed in SFZ).
-
Step down error (number of attempts made to come to shock zone).
-
Total time spent in the shock zone.
Statistical analysis
Values reported are mean ± SEM (number of animals). The significance of difference with respect to controls was evaluated using the Mann–Whitney U test. A probability (P-value) level less than 0.05 was considered as significant.
Results
Antinociception, Anticonvulsant, Motor co-ordination, Sedative–hypnotic potentiation and Anti-anxiety effects of dried latex of the aerial parts of EP (250 mg/kg, 500 mg/kg and 1000 mg/kg) were evaluated in this study and the effects were compared with vehicle control and standard control.
Effect EP on Nociception
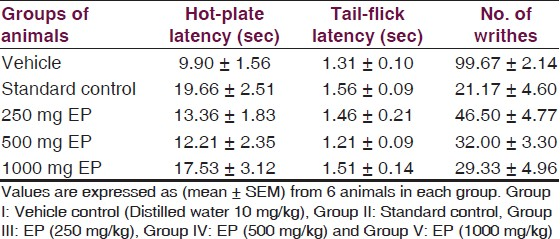
The antinociceptive activity of EP determined by using Hot plate test, Tail flick test and Acetic acid induced writhing was evaluated in three different doses of EP (250, 500 and 1000 mg/kg). EP when administered at three doses 250, 500 and 100 mg/kg showed analgesic like activity in hot plate method. There was an increase in latency of time and it was also dose dependant. The dose of 1000 mg/kg showed statically significant (P<0.05) prolongation of latency time as compared to vehicle control group in hot plate test [Table 1, Figure 1]. The effect of EP at 1000 mg/kg was comparable to that of morphine. [Table 1, Figure 1] Similarly, results were statistically indistinguishable at the three doses of EP (250, 500 and 1000 mg/kg) (P>0.05) as compared to morphine.
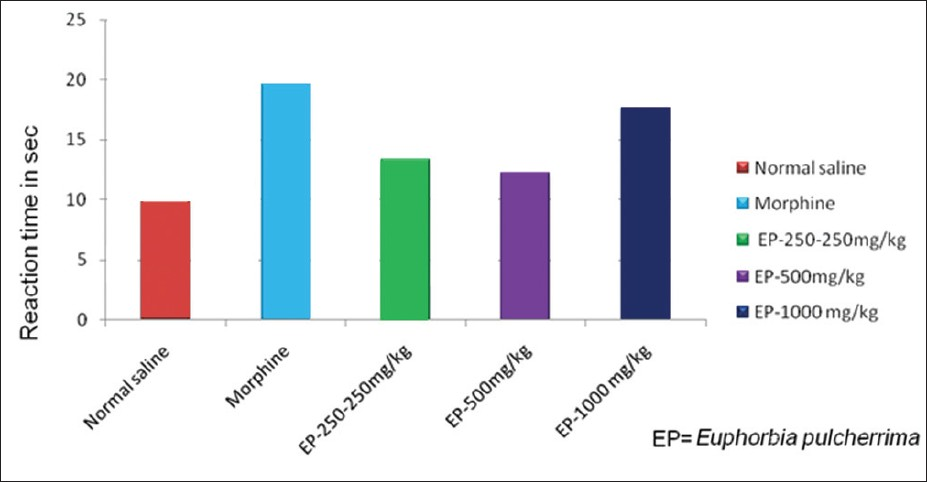
- Responses of EP in Hotplate test
In the tail flick test model, there was little difference between vehicle control treated and test drug treated groups [Table 1, Figure 2]. Further, there were no statistically significant differences among vehicle treated group and EP treated groups [Figure 2, Table 1] and the difference was also not significant compared to morphine (P>0.05).

- Responses of EP in Tail-flick test
In Acetic acid induced writhing, EP significantly (P<0.05) reduced the number of writhings produced by administration of 0.6% acetic acid as compared to vehicle treated group [Table 1, Figure 3]. EP elicited a dose dependent inhibition of abdominal constrictions as compared with the vehicle control group [Table 1, Figure 3]. The inhibition produced by EP at 1000 mg/kg was comparable to Indomethacin treated group [Table 1, Figure 3] but was statistically indistinguishable (P>0.05) as compared to indomethacin.
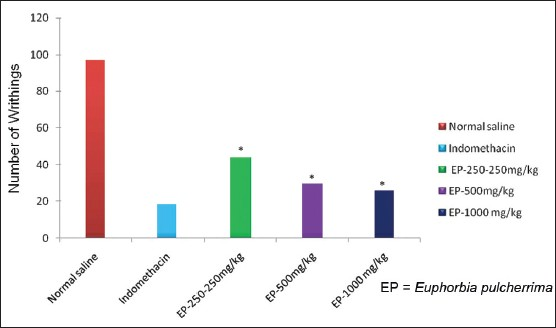
- Responses of EP in Acetic acid writhing test, *=P-value level < than 0.05
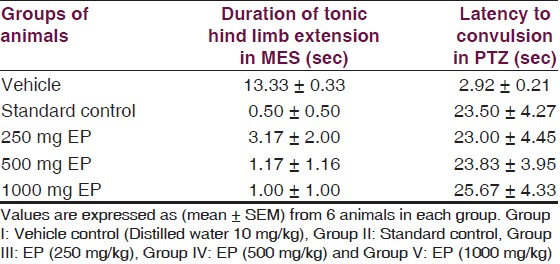
Effect of EP on convulsions: In MES test, treatment with EP in three different doses reduced the number of convulsions in animals, which suggests a protective effect in mice against MES induced convulsions. The duration of tonic hind limb extension in mice treated with EP was significantly (P<0.05) less as compared to vehicle treated group [Table 2, Figure 4]. EP was most effective at a dose of 1000 mg/kg. The duration of tonic hind limb extension with EP at 1000 mg/kg was close to that of Phenytoin [Table 2, Figure 4]. Effects of EP at 250, 500 mg/kg and 1000 mg/kg is statistically indistinguishable (P>0.05) as compared to Phenytoin at 10 mg/kg.
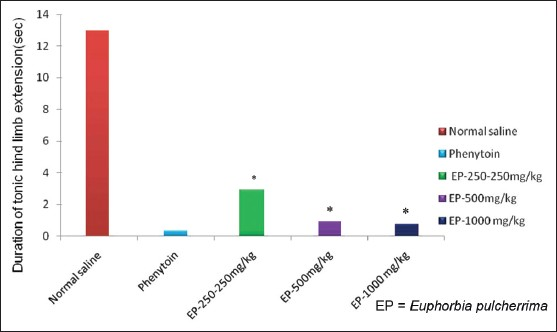
- Responses of EP in MES Test
Likewise, there was also an increase in the latency of convulsions with the use of EP in three different doses in PTZ induced seizure [Table 2]. EP in all three doses significantly (P<0.05) increased the latency and decreased the incidence of convulsions induced by PTZ seizures [Table 2, Figure 5]. Also, effects of EP in three different doses in PTZ induced seizures was statistically indistinguishable (P>0.05) as compared to Valproic acid 300 mg/kg.
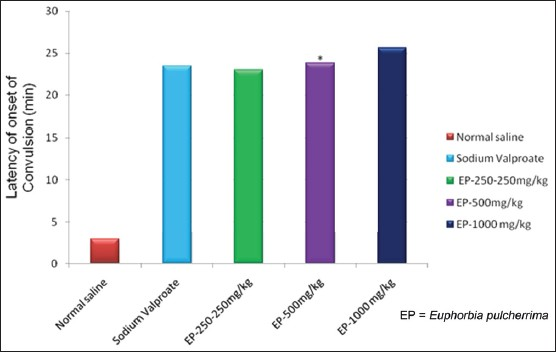
- Responses of EP in PTZ induced seizures test, *=P-value level < than 0.05
Effect of EP on motor co-ordination: Rota-rod tread mill device was used to evaluate the effect on motor co-ordination. Administration of EP in three different doses did not induce significant (P>0.05) motor incoordination [Table 3, Figure 6] as compared to vehicle control. But, results of EP in three different doses are statistically significant (P<0.05) as compared to Diazepam (5 mg/ kg) except endurance time at 2 hr at doses of 500 and 1000 mg/kg.
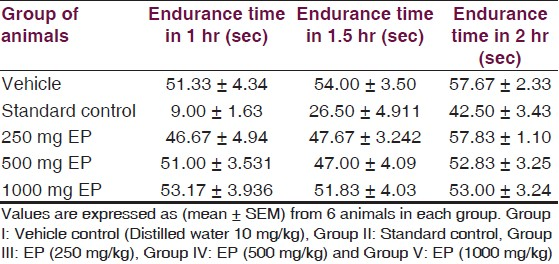
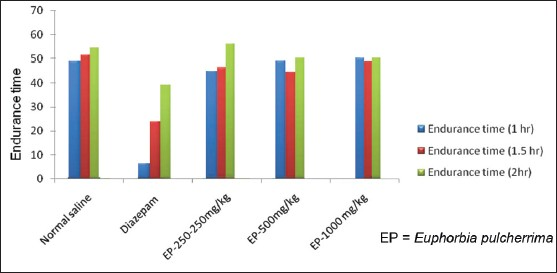
- Responses of EP in Motor co-ordination (Rota - rod) test, *=P-value level < than 0.05
Effect of EP on Pentobarbital induced sleeping time: Sedation in rats was induced by i.p. administration of 40 mg/kg of pentobarbital. EP in three different doses did not increase total sleeping time as compared to vehicle control treated group [Table 4, Figure 7].
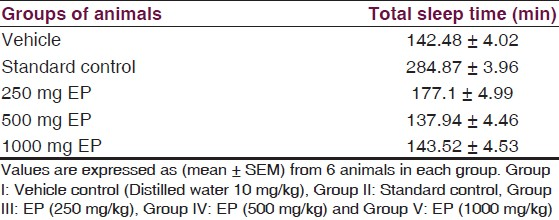
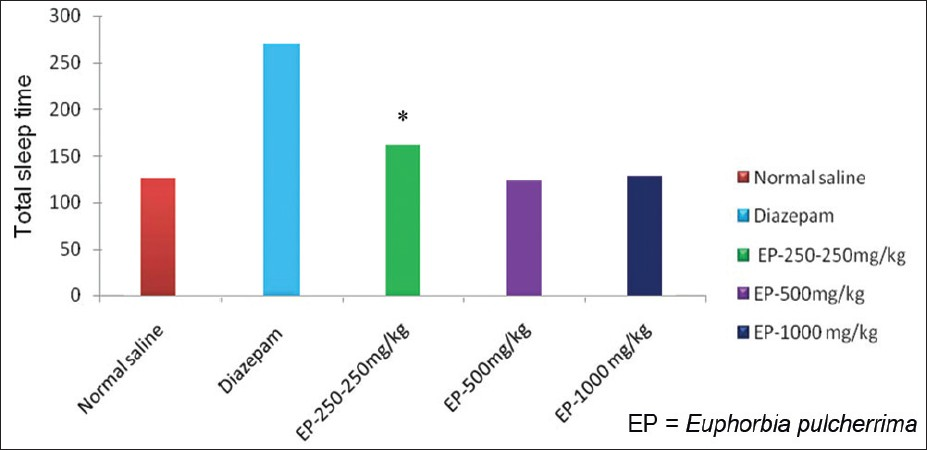
- Responses of EP in pentobarbital induced sleeping time, *=P-value level < than 0.05
Effect of EP on behavior models: In Open-field test, there was no significant alteration (P>0.05) in the parameters (number of squares crossed, time spent in central square and number of rearing) with EP in three different doses as compared to control group [Table 5, Figure 8] suggesting that EP has no effect on anti-anxiogenic-like activity and locomotion.
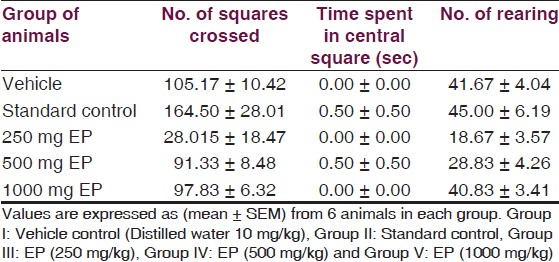
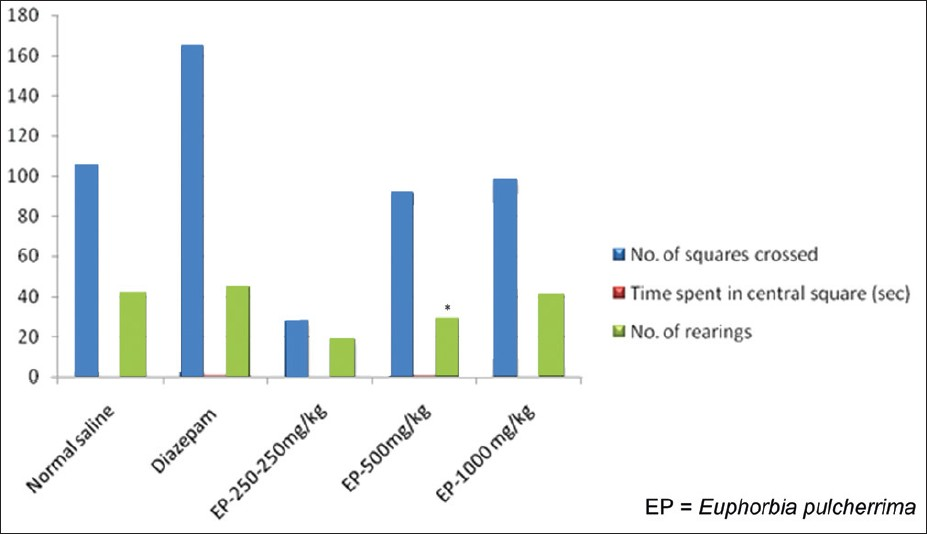
- Responses of EP in Open-field test, *=P-value level < than 0.05
In passive avoidance test, no significant effects were seen on step down latency, step down error and time spent in shock zone [Table 6, Figure 9]. This result showed that EP in three different doses did not have effect on learning and memory.
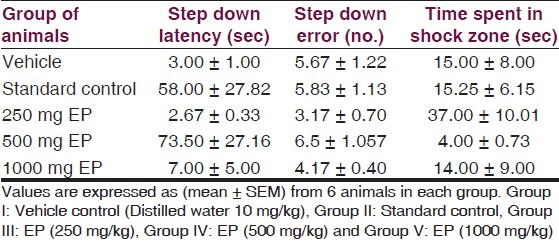

- Responses of EP in Passive avoidance test, Effects of Euphorbia pulcherrima (EP) on experimental models
Discussion
The present study demonstrates the neuropharmacological effects of crude dried latex of Euphorbia pulcherrima (EP) on mice and rats using various experimental models.
Antinociceptive, anticonvulsant, sedative–hypnotic and anti-anxiety effects of crude dried latex of the aerial parts of EP were evaluated in this study. The outcome of the present study demonstrates that EP produces several effects on the CNS.
In preliminary experiments, the toxicity of crude extract of EP was tested, and judged from the high doses (1500 mg/kg) tolerated without significant overt mortality or signs of toxicity. It was estimated that the doses used in the experiment may reflect the low concentration of the active compound present in the crude extract.
To our knowledge, this is the first report on the neuropharmacological aspects of EP. However, there are previous reports on the CNS depressing activity of other related Euphorbia species. For example, E. neriifolia leaf extract had anti-anxiety, anti-psychotic and anticonvulsant action,[5] E. hirta was reported to have sedative and anxiolytic effect,[67] and E. fisheriana produced significant antiepileptic effect in patients having intractable epilepsy.[10]
In the present study, EP was studied for its antinociceptive activity in both peripheral and central analgesic models. The antinociceptive tests used in the present work were chosen in order to test different nociceptive stimuli namely thermal (hotplate), radiant (tail flick) and chemical visceral nociceptive stimuli (acetic acid). It was essential to employ more than one test to confirm the antinociceptive action because it has been shown that some ‘false positive’ activity can be observed with agents that are not normally considered as analgesic.[28] It was found that EP crude dried extracts administered in three doses (250, 500 and 1000 mg/kg) were effective in producing analgesic activity in hot plate test but statistically not significant compared to vehicle control. In the tail flick model, analgesic activity was not seen. In acetic acid induced writhing, EP (250, 500 and 1000 mg/kg) significantly reduced the number of writhings in acetic acid test. Further, EP also produced a dose dependent inhibition of abdominal constrictions. The intensity of antinociceptive effect of EP at 1000 mg/kg dose was similar to that of indomethacin (20 mg/kg, PO) in the acetic acid model.
Pain is a subjective experience, which is difficult to define exactly. Pain is distinguished as two types, peripheral and neurogenic/central pain which may involve the following pathological states: peripheral nociceptive afferent neurons, which are activated by noxious stimuli and central mechanism, which is activated by afferent input pain sensation.[29] The hot plate and tail flick tests are considered to be selective for opioid-like compounds, which are centrally acting analgesics in several animal species.[30] The increase in the reaction time of the mice on the hot plate following administration of the extracts suggests that the extracts possess central analgesic activity. The suppression of the acetic acid-induced writhing suggests, however, that the extracts may act via local peritoneal receptors.[31] The intraperitoneal injection of acetic acid produces pain through the activation of chemosensitive nociceptors or irritation of the visceral surface, thereby leading to the liberation of bradykinin, histamine, prostaglandins and serotonin. Acetic acid causes inflammatory pain by inducing capillary permeability[32] and liberating endogenous substances that excites pain nerve endings.[33] Nonsteroidal anti-inflammatory drugs (NSAIDs) can inhibit cyclooxygenase enzyme (COX) in peripheral tissues and, therefore, interfere with the mechanism of transduction of primary afferent nociceptors.[34]
The extract inhibited the pain induced by acetic acid which indicates that EP act through both mechanisms, i.e. central, as well as peripheral analgesics.[3536] Therefore, it can be inferred that the extract may have produced antinociception via both central and peripheral mechanisms.
However, the mechanism of this action has not been investigated here. It is not known whether this action is opioid-like in nature and/or involves acetylcholine or other agents. The use of selective antagonists (e.g. Naloxone or Atropine) might delineate this. The mechanism of analgesic effect of EP in acetic acid induced writhing could be due to blockade of the effect or the release of endogenous substances that excites pain nerve endings similar to that of indomethacin and other NSAIDs.[3537]
‘False positive’ activity has been reported in acetic acid induced writhing test.[28] The three tests used did not confirm the antinociceptive action of EP. It is possible that the extract contained several chemical constituents that exert more than one action via different mechanisms.
To evaluate anticonvulsive properties of EP, PTZ induced seizure and MES models were used. PTZ is the most frequently used substance, as well as an acute experimental model, in preliminary screening to test potential anticonvulsant drugs.[38] PTZ is believed to exert its action is by acting as an antagonist at the γ-aminobutyric acid type A (GABAA) receptor complex.[39] Drugs protecting against tonic-clonic seizures induced by PTZ are considered to be useful to control myoclonic and absence seizures in humans.[40] Ethosuximide, a blocker of T-type Ca2+ currents, can prevent PTZ-induced seizures and is effective in controlling absence epilepsy, most commonly in childhood.[41] The drugs which inhibit the MES induced seizures mostly inhibit the sodium current and blocks the repetitive firing of neurons.[42] It has often been stated that seizures induced by PTZ, can be blocked by drugs that enhance GABAA receptor-mediated inhibitory neurotransmission, such as benzodiazepines and phenobarbital.[4344]
In the present study, EP statistically significant inhibited the MES induced seizures and PTZ induced seizures in mice. This may suggest that the anticonvulsant action of EP is mediated through the GABAA/benzodiazepine receptor complex. The formulation may act by increasing GABA concentration in the brain because PTZ is a known GABAA receptor antagonist.[45] The benzodiazepine site in the GABAA receptor and even T type Ca2+ currents could be targets for future studies to know mechanisms of action of the EP extract and/or its components. In our experiment, the extract of EP replicated the effect of this antiepileptic drug delaying the presence of seizures and reducing tonic convulsions and mortality.
The prolongation of the onset time of PTZ convulsions by EP extract indicates anticonvulsant effect of the plant, which again involves the inhibition of excitatory mechanisms in the CNS, although the parameters i.e. onset time of convulsions, decreased duration of tonic clonic convulsion used for evaluation of anticonvulsant activity in the present study are not conclusive. However, it gives a preliminary indication about the anticonvulsant effect of EP extract. Further studies are needed to answer questions like the differential effects on tonic and clonic convulsions; effect on mortality following the PTZ induced convulsions and preventive effects of the plant on convulsions during chronic administration.
Conclusion
In conclusion, this study evaluated various central nervous system effects of Euphorbia pulcherrima crude dried extracts administered in three doses (250, 500 and 1000 mg/kg) using various animal models. This is in our knowledge, the first experimental study for EP to characterize its neuropharmacological profile. But a few isolated studies for other Euphorbia species have also been reported. This study showed EP crude dried extracts to possess anticonvulsant and antinociceptive properties but no effect on models of motor co-ordination and anxiety (open field test and passive avoidance test). This study provides evidence supporting anticonvulsive properties of other related Euphorbia species.
There is a need for more precise studies to determine and separate the active compounds and elucidate their mechanisms of action where possible. So, further studies are needed to determine the exact mechanism(s) of action for anticonvulsant and antinociceptive action of the various compounds in the crude extract of EP.
The authors would like to acknowledge the help of Prof. Dr. S.K Bhattacharya, University College of Medical Sciences, Delhi, India for his constructive criticism and help in accomplishing this study. They are also thankful to Prof. Dr. P. Ravi Shankar, KIST Medical College, Lalitpur, Nepal for proof reading the manuscript. They acknowledge the help of the supporting staff members Mr. Gokarna Bhandari, Mrs. Rusha Tamrakar, Mr. Om Prakash Chaudhary and Mr. Harka Pakhrin.
Source of Support: Nil
Conflict of Interest: None declared
References
- Plants that heal. Pune, India: Oriental Watchman Publishing House; 1994. p. :134-56.
- Analgesic, anti-pyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 1991;57:225-31.
- [Google Scholar]
- Activity on CNS crude extracts and of some diterpenoids isolated from Euphorbia calyptrate suspended cultures. Planta Med. 1991;57:531-5.
- [Google Scholar]
- Psychopharmacological profile of hydro-alcoholic extract of Euphorbia neriifolia leaves in mice and rats. Indian J Exp Biol. 2005;43:859-62.
- [Google Scholar]
- Behavioral effects of Euphorbia hirta: Sedative and anxiolytic properties. Phytother Res. 1996;10:670-6.
- [Google Scholar]
- Behavioral effects of Euphorbia hirta: Sedative and anxiolytic properties. J Ethnopharmacol. 1990;29:189-98.
- [Google Scholar]
- Influence of some traditional medicinal plants of Senegal on prostaglandin biosynthesis. J Ethnopharmacol. 1994;42:111-6.
- [Google Scholar]
- Analgesic and antipyretic properties of Euphorbia royleana latex. Phytother Res. 1997;11:597-9.
- [Google Scholar]
- Clinical analysis of 72 epileptic patients treated with alkaline extract of Euphorbia fisheriana. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14:282-4. 261
- [Google Scholar]
- Chemical study of the latex, stems, bracts, and flowers of “Christmas Flower” (Euphorbia pulcherrima) J Pharm Sci. 1967;56:1184-5.
- [Google Scholar]
- Toxicity of fresh poinsettia (Euphorbia pulcherrima) to Sprague-Dawley rats. Clin Toxicol. 1980;16:167-73.
- [Google Scholar]
- Synthetic analgesics: II. Dithienylbutenyl and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385-93.
- [Google Scholar]
- Radiant heat and hot-plate method. In: Drug discovery and evaluation. Berlin Heidelberg Germany: Springer - Verilog; 1997. p. :694-7.
- [Google Scholar]
- Comparison of antiepileptic drugs Tiagabine, Lamotrigine and Gabapentin in mouse models of acute, prolonged and chronic nociception. J Pharmacol Exp Ther. 2002;302:1168-75.
- [Google Scholar]
- Analgesic activity of acetyl-11-keto-beta-boswellic acid, a 5-lipoxygenase-enzyme inhibitor. Indian J Pharmacol. 2005;37:255-6.
- [Google Scholar]
- The abdominal response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295-310.
- [Google Scholar]
- The type of analgesic-receptor interaction involved in certain analgesic assays. Eur J Pharmacol. 1971;16:63-6.
- [Google Scholar]
- Anticonvulsants. In: Laurence DR, Bacharach AL, eds. Evaluation of drug activities, Pharmacometrics. Vol 1. London and New York: Academic Press; 1964. p. :290.
- [Google Scholar]
- Thioperamide, selective histamine H3 receptor antagonist, protects against PTZ-induced seizures in mice. Life Sci. 2000;66:297-301.
- [Google Scholar]
- Potentiation of barbital hypnosis as an evaluation method for central nervous system depressant. Psychopharmacologia. 1965;7:374-8.
- [Google Scholar]
- Experimental methods for evaluation of psycho- tropic agents in rodents: I- Antianxiety agents. Indian J Exp Biol. 1997;35:565-75.
- [Google Scholar]
- A note on “stretched attention”, a behavioral element indicative of an approach-avoidance conflict in rats. Anim Behav. 1979;27:446-50.
- [Google Scholar]
- Open field behavior in the rats. What does it mean? Ann N Y Acad Sci. 1969;159:852-9.
- [Google Scholar]
- Effect of cholinergic drugs on passive avoidance in the mouse. J Pharmacol Exp Ther. 1967;158:279-85.
- [Google Scholar]
- Comparative analgesic testing of various compounds in mice using writhing techniques. Arzneimittelforschung. 1978;28:1644-7.
- [Google Scholar]
- Pharmacology (5th ed). Edinburgh: Churchill Livingstone Publication; 2005. p. :562.
- The inhibitory effects of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Arzneimittelforschung. 1963;13:502-7.
- [Google Scholar]
- Pharmacological assays. In: Drug discovery and evaluation, 402-41. Germany: Springer - Verlag Berlin Heidelberg; 1997. p. :370.
- [Google Scholar]
- Gangliosides antinociceptive effects in rodents. Arch Int Pharmacodyn Ther. 1984;272:103-17.
- [Google Scholar]
- Pain mechanism. In: Raj PP, ed. Pain medicine: A comprehensive review (1st ed). Missouri: Mosby-Year Book; 1996. p. :12-23.
- [Google Scholar]
- Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mousemacrophage RAW264.7 cells. Life Sci. 2003;73:337-48.
- [Google Scholar]
- Intraplantar injection of dextrophan, ketamine or memantine attenuates formalin induced behaviors. Brain Res. 1998;785:136-42.
- [Google Scholar]
- Dehydroisoeugenol, an isoeugenol dimer, inhibits lipopolysaccharide-stimulated nuclear factor kappa B activation and cyclooxygenase-2 expression in macrophages. Arch Biochem Biophys. 2005;434:326-32.
- [Google Scholar]
- Experimental selection, quantification and evaluation of anticonvulsants. In: Antiepileptic Drugs (3rd ed). New York: Raven Press; 1989. p. :85-102.
- [Google Scholar]
- Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor ionophore complex. Eur J Pharmacol. 1984;98:337-45.
- [Google Scholar]
- Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145-81.
- [Google Scholar]
- Contemporary diagnosis and management of the patient with epilepsy (5th ed). Newtown, Pennsylvania, USA: Handbooks in Health Care Company; 2001. p. :9-34.
- Drugs effective in the therapy of the epilepsies. In: Ruddon RW, Molinoff PB, Limbird LE, Hardman JG, eds. The Pharmacological basis of Therapeutics (10th ed). New York: McGraw-Hill; 2001. p. :521-47.
- [Google Scholar]
- Antiepileptic drugs and pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990;42:223-86.
- [Google Scholar]
- Anticonvulsive activity of Albizzialebbeck, Hibiscus rosasinesis and Buteamonosperma in experimental animals. J Ethnopharmacol. 2000;71:65-75.
- [Google Scholar]






