Translate this page into:
Effect of fluoride exposure on the intelligence of school children in Madhya Pradesh, India
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To assess the relationship between exposure to different drinking water fluoride levels and children's intelligence in Madhya Pradesh state, India.
Materials and Methods:
This cross-sectional study was conducted among 12-year-old school children of Madhya Pradesh state, India. The children were selected from low (< 1.5 parts per million) and high (≥1.5 parts per million) fluoride areas. A questionnaire was used to collect information on the children's personal characteristics, residential history, medical history, educational level of the head of the family, and socioeconomic status of the family. Levels of lead, arsenic, and iodine in the urine and the levels of fluoride in the water and urine were analyzed. The children's intelligence was measured using Raven's Standard Progressive Matrices. Data analysis was done using the chi-square, one way analysis of variance, simple linear regression, and multiple linear regression tests. P value <0.05 was considered statistically significant.
Results:
Differences in participant's sociodemographic characteristics, urinary iodine, urinary lead, and urinary arsenic levels were statistically not significant (P>0.05). However, a statistically significant difference was observed in the urinary fluoride levels (P 0.000). Reduction in intelligence was observed with an increased water fluoride level (P 0.000). The urinary fluoride level was a significant predictor for intelligence (P 0.000).
Conclusion:
Children in endemic areas of fluorosis are at risk for impaired development of intelligence.
Keywords
Child
fluoride poisoning
intelligence
India
water
Introduction
Fluoride is known to have both beneficial and adverse effects on humans.[1] Introduction of systemic and topical fluorides into the prevention and control of dental caries denotes the most significant issue in dentistry. Fluorides have brought a considerable decline in the prevalence of dental caries, especially in countries with advanced economies.[2] However, in the 1980s it was established that fluoride controls caries mainly through its topical effect.[3] Drinking water is usually, but not always, the main source of fluoride.[1] Elevated concentration of naturally occurring fluoride in drinking water is a worldwide problem. Many Asian countries including India have reported a concentration of fluoride exceeding the World Health Organization (WHO) guidelines values or their prevailing national standards.[4] Sixty million Indians are living in about 200 districts of 20 states in endemic areas of fluorosis.[5] Dental and skeletal effects associated with fluoride, in humans, are well documented.[45] Also, the existing literature reports the neurological consequences associated with exposure to fluoride. In children, most reported effects are on the cognitive capacities, particularly intelligence reduction.[46–11] On the other hand studies conducted by Hu et al.[8] and Spittle et al.[12] have not found any trend or correlation between fluoride and the intelligence of children.
The majority of studies that show a correlation between a lower Intelligence Quotient (IQ) and elevated fluoride intake are from China. To the best of our knowledge, there is only one piece of literature[9] published from the Indian subcontinent, which shows that fluoride exposure has an effect on the intellectual function of children. Many of the published studies have methodological limitations, like no consideration for the effect of lead, arsenic, iodine deficiency, socioeconomic status (SES) or nutritional status of the children.
Keeping in mind the above-mentioned confounders, the current study has been designed to assess the relationship between exposure to different drinking water fluoride levels and children's intelligence in Madhya Pradesh state, India.
Materials and Methods
Prior to the study, a detailed study protocol was submitted to the Ethics Committee of the Institution. After obtaining the ethical approval, a pilot study was conducted in high and low fluoride areas. Based on the results of a pilot study, to achieve a 5% level of significance and 80% power, the sample size for the present study was calculated using Power and Precision v. 4 (Biostat Inc., Englewood, USA).
Study population
This cross-sectional study was conducted among 12-year-old school children of Madhya Pradesh state, India. The subjects were selected by a stratified cluster sampling of areas, according to the fluoride concentration in the groundwater (<1.5 parts per million (PPM) and ≥1.5 PPM), based on the geological survey report of the Government of India.[1314]
On the basis of the report, 120 school children of the villages of Karera Block, Shivpuri district, Madhya Pradesh state, India, were included in the high fluoride group (water fluoride level ≥1.5 PPM). Children of this group were further sub-divided into the following three groups, after fluoride analysis of their drinking water; (1) 1.5 – 3.0 PPM (n=39), (2) 3.1 – 4.5 PPM (n=43), and (3) >4.5 PPM (n=38). Fifty school children from Parwaliya village, Bhopal district, Madhya Pradesh state, India, were included in the low fluoride group (water fluoride level <1.5 ppm). The selected villages were similar in population and general demographic characteristics. The selected children were from government schools and were in the fifth or sixth class.
Participation in the study was voluntary. Informed consent for the same was obtained from the parents and children.
Children who were not lifelong residents of that area, those who had a change in source of water since birth, and had a history of congenital or acquired neurological disease and/or head injury, were excluded from the study.
A questionnaire, completed with the assistance of parents, was used to collect information on the personal characteristics (age, sex, height, weight), residential history, medical history, including illness affecting the nervous system and head trauma, educational level of the head of the family (in years), and SES of the family. The SES was recorded according to the Pareek and Trivedi classification.[15] The Pareek and Trivedi scale graded the SES into three categories; (1) Low (2) Middle, and (3) High.
The nutritional status of the children was calculated using the Waterlow's classification.[16] It defines two groups for malnutrition; (1) Height for age ratio (chronic condition) (2) Weight for Height (acute condition). In both the groups it categorizes the malnutrition as; (1) Normal, (2) Mildly impaired, (3) Moderately impaired, and (4) Severely impaired.
Water sample collection and analysis: A sample of 200 ml of drinking water was collected in a polyethylene bottle at each child's home. The fluoride levels were analyzed by a fluoride ion selective electrode, Orion 9609BN (Thermo Fisher Scientific Inc., West Palm Beach, United States).
Urine sample collection and analysis: Each subject was also asked to collect a sample of their first morning urine. The fluoride content in the urine was determined using a fluoride ion selective electrode, Orion 9609BN (Thermo Fisher Scientific Inc., West Palm Beach, United States). Lead and Arsenic were analyzed using the atomic absorption spectrophotometer (Perkin-Elmer, Wellesley, United States). Urinary iodine was measured using the Dunn method.[17]
Assessment of intelligence: Children's intelligence was measured using the Raven's Standard Progressive Matrices.[18] The children's scores were converted to percentile and specific grades were allotted, based on the following criteria:
Grade I: Intellectually superior — If the score lies at or above the ninety-fifth percentile for that age group.
Grade II: Definitely above average — If the score lies at or above the seventy-fifth percentile for that age group.
Grade III: Intellectually average — If the score lies between the twenty-fifth and seventy-fifth percentile for that age group.
Grade IV: Definitely below average in intellectual capacity — If the score lies at or below the twenty-fifth percentile for that age group.
Grade V: Intellectually impaired — If the score lies at or below the fifth percentile for that age group.
As stated above, the grades and intelligence are inversely related in the Raven's Standard Progressive Matrices.
Statistical analysis
SPSS v.17 (SPSS Inc., Chicago, USA) was used for the statistics. Data was statistically analyzed using the chi-square, one way analysis of variance, simple linear regression, and the multiple linear regression tests. P value <0.05 was considered statistically significant.
Results
The sociodemographic characteristics of the children, with different levels of water fluoride, are presented in Table 1. There were almost an equal number of boys and girls in each group. The SES was between middle and high. Mean years of education of the head of the family were seven. Height for age ratio and weight for height ratio revealed that most of them were in the mild to moderately impaired category. No statistically significant differences were observed in the participant's gender proportion, SES, education of the head of the family, height for age ratio, or weight for height ratio.

Table 2 shows the levels of urinary fluoride, iodine, lead, and arsenic in children with different levels of water fluoride. A statistically significant difference was observed in urinary fluoride levels between four groups. Differences in urinary iodine, urinary lead, and urinary arsenic were not significant.
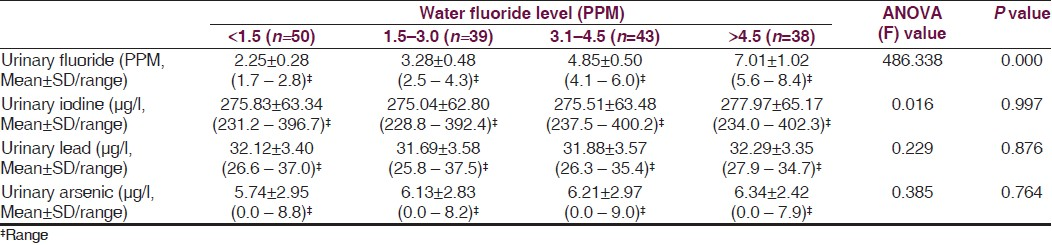
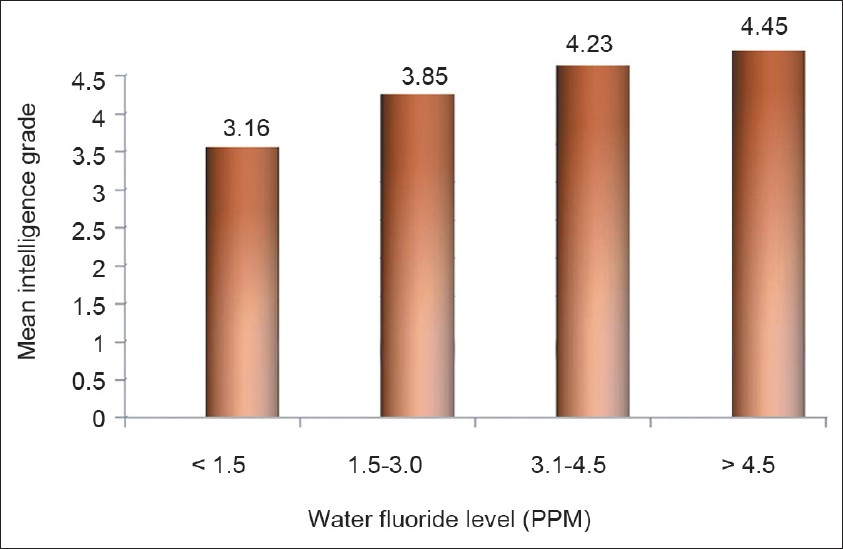
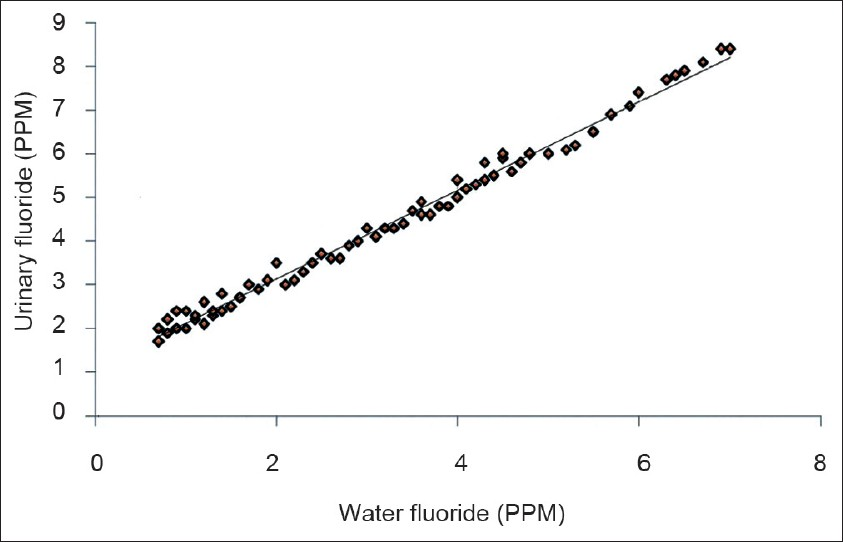
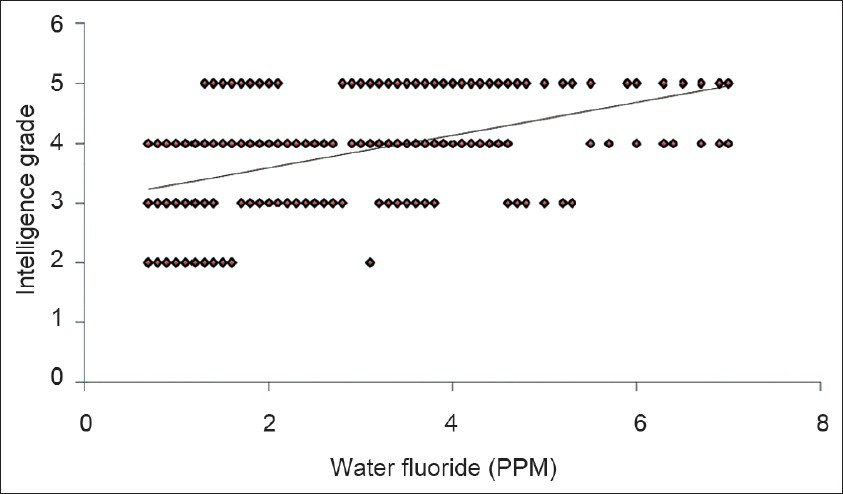
The mean intelligence grades of the children with different levels of water fluoride are presented in Figure 1. The lowest mean grade was observed with water fluoride level <1.5 PPM. The highest mean grade (worst intelligence) was observed with water fluoride level >4.5 PPM. The differences for intelligence grades were statistically significant between the four groups (ANOVA=22.130, P 0.000). Figure 2 shows simple linear regression analysis, with urinary fluoride as a dependent variable. There was a statistically significant relationship of the water fluoride level with the urinary fluoride level (R=0.995, R2 =0.991, ANOVA=17897.210, P 0.000). When simple linear regression analysis was performed, with intelligence grades as the dependent variable, a significant relationship of intelligence grades was observed with the water fluoride level [Figure 3, R=0.534, R2 =0.286, ANOVA=67.14, P 0.000] and urinary fluoride level [Figure 4, R=0.542, R2 =0.294, ANOVA=69.94, P 0.000]. However, in stepwise multiple linear regression analysis the only significant independent variable was urinary fluoride [Table 3].
- Mean intelligence grades of children with different levels of water fluoride
- Simple linear regression analysis with urinary fluoride as a dependent variable and water fluoride as an independent variable
- Simple linear regression analysis with the intelligence grade as a dependent variable and water fluoride as an independent variable
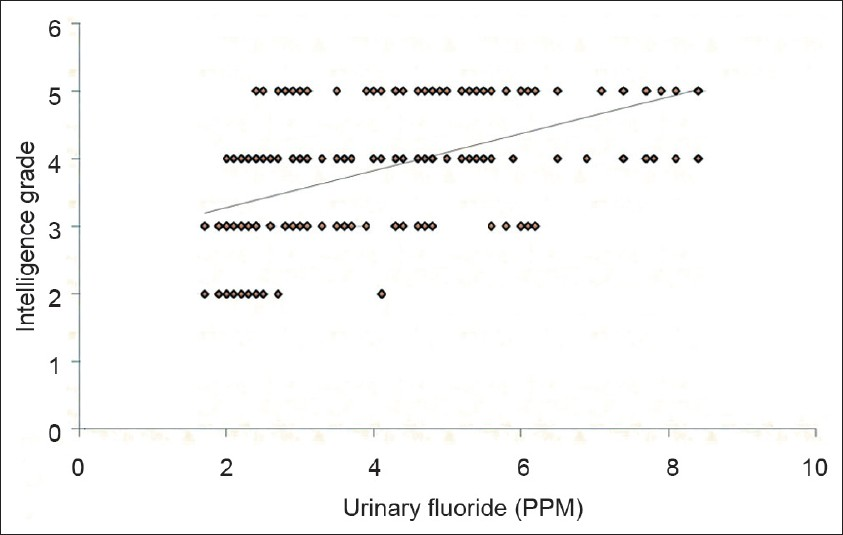
- Simple linear regression analysis with the intelligence grade as a dependent variable and urinary fluoride as an independent variable

Discussion
This study indicates that exposure to fluoride is associated with reduced intelligence in children. We have found a significant inverse relationship between intelligence and the water fluoride level, and intelligence and the urinary fluoride level. After adjusting for confounders, urinary fluoride was the significant predictor for intelligence. The kidneys are the principal organs for the excretion of fluoride. Fluoride in urine is a better indicator for exposure compared to water, as it integrates all the sources.[419] Therefore, the degree of exposure to fluoride in the present study has been checked by analyzing the urinary fluoride level.
The impact of fluoride on intelligence has been reported by a number of studies. Many of them have several shortcomings, like the lack of adjustment for confounders, no biological markers, no quantification for other neurotoxic pollutants, and so on. Despite all of that, these studies suggest that fluoride negatively impacts intelligence.[46–11]
Studies have shown that exposure to lead and arsenic has been associated with a decline in intellectual function in children.[410] Also, the education level of parents, SES of the family,[46–8] nutrition,[2021] and iodine deficiency[79] affect the intellectual ability of children. Therefore, these factors were considered in the present study. In the present study lead and arsenic in urine were within the documented safe zone (Normal human levels — lead ≤80 μg/l,[2223] arsenic ≤100 μg/l[2425] ). Moreover, differences in the urinary lead and arsenic levels of children with different levels of water fluoride were not significant. These findings indicated that the observed deficits in intelligence could not be attributed to lead and arsenic exposure. Data from previous studies could lend support to the hypothesis that interaction between fluoride, lead, and arsenic could worsen the children's intelligence grades,[4] and thus, indicate a need for further investigation. In a low iodine area, impairment of intelligence could occur through the development of hypothyroidism or clinical/subclinical cretinism.[7] In our study, we could not find a significant difference in the urinary iodine levels of children with different levels of water fluoride. Also, as per the WHO criteria, the iodine status of all the children was ‘Adequate’ (>100 μg/l).[2627] Numerous studies in animal and human models demonstrated that suboptimal nutrition in early life affects the cognitive performance.[2021] However, in present study, indices for chronic and acute malnutrition failed to show any relationship with intelligence. Studies conducted by Lu et al.[7] and Xiang et al.[8] showed that the relationship of the SES of the family and the parents’ education level, with the children's IQ, was not significant. These finding were consistent with the findings in the present study. However, Zaho et al. stated that the children's IQ increased with parents education level.[6] In a Mexican study by Rocha-Amador et al., after adjusting for confounders (blood lead, SES, mothers’ education, nutritional status), the fluoride in urine was associated with reduced IQ scores.[4] In a meta analysis Tang et al. concluded that children who lived in an endemic fluoride area had five times higher odds of developing low IQ than those who lived in a non-fluoride area.[11]
It is very well established that fluoride can penetrate the blood brain barrier.[6–91128] Also, it can pass through the placenta to the fetus,[6–91129] and with subsequent continuous exposure to fluoride during childhood, it may have adverse effects on the developing brain, thereby causing decreased intelligence in children.[6–911] The biomechanism of the action of fluoride in reducing intelligence is still not clear. However, there is evidence that it may involve the alteration of the membrane lipid and cause a reduction in the cholinesterase activity in the brain. This may lead to altered utilization of acetylcholine, affecting the transmission of nerve impulses in the brain tissue.[30–32] NaF has been found to alter the levels of dopamine, serotonin, 5-hydroxyindoleacetic acid, homovanille acid, norepinephrine, and epinephrine, in the hippocampus and neocortex regions of the rat brain.[33] Yu et al. demonstrated changes in neurotransmitters and their receptors in the fetal brain from the endemic fluorosis area.[7] Thyroid hormones play an important role in the development of the brain. In a study, Susheela et al. found that elevated fluoride uptake may cause iodine deficiency in fluorotic individuals, even when they reside in non-iodine deficient areas.[34]
Limitations: In this cross-sectional study, we have analyzed the fluoride in urine, a biomarker of recent exposure. We have not analyzed for historical markers such as dental fluorosis. We assume that the exposure scenario has not changed over time and current exposure to fluoride in drinking water can be used as a proxy for past exposure. We have analyzed a single morning urine sample, instead of a 24-hour urine sample. However, the studies of Villa et al.[35] and Opydo-Szymaczek and Borysewicz-Lewicka[36] reveal that there is a close relationship between the concentration of fluoride in the first morning sample and in the 24-hour specimen. Children's intelligence can be influenced by inheritance. Children in our study groups attend school and are therefore exposed to different levels of fluoride, while not at home. Although the children who had changed their water source since birth were excluded in the present study, we could not completely exclude the influence of recall bias. Moreover, fluoride level in a particular water source may change over a period of years. Therefore, we emphasize the need for a more careful evaluation of the effect of fluoride on intelligence.
In conclusion, data from this research supports that children exposed to fluoride are at risk for impaired development of intelligence. Millions of children around the world are exposed to high concentration of fluoride in water, and are therefore, potentially at risk. For the benefit of the upcoming generations, urgent attention needs to be focused on this substantial public health problem.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Exposure to high fluoride drinking water and risk of dental fluorosis in Estonia. Int J Environ Res Public Health. 2009;6:710-21.
- [Google Scholar]
- Is systemic fluoride supplementation for dental caries prevention in children still justifiable? Prague Med Rep. 2007;108:306-14.
- [Google Scholar]
- Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97-114.
- [Google Scholar]
- Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saude Publica. 2007;23(Suppl 4):S579-87.
- [Google Scholar]
- Effect of a high fluoride water supply on children's intelligence. Fluoride. 1996;29:190-2.
- [Google Scholar]
- Effect of fluoride in drinking water on children's intelligence. Fluoride. 2003;36:84-94.
- [Google Scholar]
- Effect of high fluoride water on intelligence of school children in India. Fluoride. 2007;40:178-83.
- [Google Scholar]
- Arsenic and fluoride exposure in drinking water: Children's IQ and growth in Shanyin county, Shanxi Province, China. Environ Health Perspect. 2007;115:643-7.
- [Google Scholar]
- Fluoride and Children's Intelligence: A Meta-analysis. Biol Trace Elem Res. 2008;126:115-20.
- [Google Scholar]
- Central Ground Water Board. Ministry of Water Resources, Govt of India. North Central region Bhopal. Available from: http://cgwb.gov.in/ncr/geochemical.htm
- [Google Scholar]
- Shivpuri district - Central Ground Water Board. Available from: http://cgwb.gov.in/District_Profile/MP/Shivpuri.pdf
- [Google Scholar]
- Manual of socio-economic status scale (rural). New Delhi: Manasayan Publisher; 1995.
- Park's textbook of preventive and social medicine (20th ed). Jabalpur: M/s Banarsidas Bhanot; 2009.
- Raven manual: Section 3. The standard progressive matrices. Oxford: Oxford Psychologists Press; 1992.
- Excess fluoride ingestion and thyroid hormone derangements in children living in Delhi, India. Fluoride. 2005;38:98-108.
- [Google Scholar]
- Effect of Ascaris infection on the nutritional status and I.Q. of children. Int J Anthropol. 1999;14:55-9.
- [Google Scholar]
- The effect of early human diet on caudate volumes and IQ. Pediatr Res. 2008;63:308-14.
- [Google Scholar]
- Determination of cadmium and lead in human urine by STAT-FAAS after enrichment on activated carbon. J Anal At Spectrom. 1999;14:275-8.
- [Google Scholar]
- Harper's Biochemistry (25th ed). Stamford Connecticut: Appleton and Lange; 2000. p. :869.
- Criteria for case definition of Arsenicosis. In: Chappell WR, Abernathy CO, Calderon RC, Thomas DJ, eds. Arsenic Exposure and Health Effects V (1st ed). Amsterdam: Elsevier B.V; 2003. p. :117-34.
- [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) 2007. U.S. Department of Health and Human Services, Public Health Services. Toxicological Profile for Arsenic, Atlanta. Available from: http://www.atsdr.cdc.gov/toxguides/toxguide-2.pdf?id=21andtid=3
- [Google Scholar]
- Identification of iodine deficiency in the field by the rapid urinary iodide test: Comparison with the classic Sandell-Kolthoff reaction method. Thyroid. 2002;12:407-10.
- [Google Scholar]
- World Health Organization. In: Assessment of iodine deficiency disorders and monitoring their elimination: A guide for programme managers (3rd ed). Geneva: WHO Press; 2007. p. :28.
- [Google Scholar]
- Transplacental passage of fluoride in pregnant Polish women assessed on the basis of fluoride concentrations in maternal and cord blood plasma. Fluoride. 2007;40:46-50.
- [Google Scholar]
- Influence of chronic fluorosis on membrane lipids in rat brain. Neurotoxicol Teratol. 1998;20:537-42.
- [Google Scholar]
- Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride. 2000;33:17-26.
- [Google Scholar]
- Pre and post natal exposure of fluoride induced oxidative macromolecular alterations in developing central nervous system of rat and amelioration by antioxidants. Neurochem Res. 2010;35:1017-28.
- [Google Scholar]
- Dose-dependent effects of fluoride on neurochemical milieu in the hippocampus and neocortex of rat brain. Fluoride. 2007;40:101-10.
- [Google Scholar]
- Excess fluoride ingestion and thyroid hormone derangements in children living in Delhi, India. Fluoride. 2005;38:151-61.
- [Google Scholar]
- The fractional urinary fluoride excretion in young children under stable fluoride intake conditions. Community Dent Oral Epidemiol. 2000;25:344-55.
- [Google Scholar]
- Urinary fluoride levels for assessment of fluoride exposure of pregnant women in Poznan, Poland. Fluoride. 2005;38:312-7.
- [Google Scholar]






