Translate this page into:
Dengue fever complicated by spontaneous intracranial and intraspinal hemorrhage: A case report
*Corresponding author: Neena Baby, Department of Neurology, Renai Medicity Multi Super Specialty Hospital, Kochi, Kerala, India. neenaneuro2018@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Baby N, Ajith S, Koshy MS, George M, Subramaniam P, Divakar A. Dengue fever complicated by spontaneous intracranial and intraspinal hemorrhage: A case report. J Neurosci Rural Pract. 2025;16:104-8. doi: 10.25259/JNRP_275_2024
Abstract
Minor petechial hemorrhages with dengue fever are common due to thrombocytopenia. We report a case of dengue fever complicated by intracranial and intraspinal hemorrhage. A 73-year-old male with dengue fever developed seizure and encephalopathy. The initial MRI of the brain was normal. He had a further drop in platelet counts, and measures to raise platelet counts were taken. An MRI was repeated after one week, which revealed subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage, and intraventricular hemorrhage in the brain and intraspinal SAH with intradural hematoma compressing the thoracic spinal cord. A continuous lumbar drain was placed after correcting the platelet levels. Follow-up imaging showed resolution of SAH and parenchymal bleed. Dengue hemorrhagic fever can be further complicated by spontaneous intracranial or intraspinal hemorrhage. Worsening of the neurological condition should alert clinicians to be highly suspicious of this extremely rare complication of dengue fever.
Keywords
Case report
Dengue encephalitis
Dengue hemorrhagic fever
Intradural hemorrhage
Intramedullary hemorrhage
Intraspinal hemorrhage
Subarachnoid hemorrhage
INTRODUCTION
Dengue is among the most common arboviral illnesses across the tropical and subtropical regions of the world, with clinical presentations ranging from asymptomatic to aggressive dengue hemorrhagic fever. Subarachnoid hemorrhage (SAH) and spinal intradural hemorrhage are extremely rare in dengue fever, and only a few cases have been reported so far. We report a rare case of spontaneous intracranial and intradural hemorrhage in dengue fever associated with thrombocytopenia.
CASE REPORT
A 73-year-old male with known systemic hypertension, type 2 diabetes mellitus, and dyslipidemia presented with generalized tiredness for two days. Clinical examination revealed that he was conscious, mild confusion was noted, and there was no focal neurological deficit.
Diagnostic assessment
His initial blood parameters showed hyponatremia, thrombocytopenia, leukopenia, anemia, and deranged blood sugars. The dengue non-structural protein 1 antigen and immunoglobulin M antibody tests turned positive. He developed an episode of seizure followed by reduced responsiveness; hence, the possibility of dengue encephalopathy was considered. An electroencephalogram done showed mild slowing and triphasic waves. C-reactive protein, procalcitonin, and urine routine tests were all normal. A magnetic resonance imaging (MRI) brain done, showed no features to suggest dengue encephalitis. Abdominal ultrasonography showed features of acalculous cholecystitis. Serial platelet counts showed a declining pattern and had dropped to a level of 45,000/mm3, platelet concentrate transfusion was given. He was given levetiracetam, broad-spectrum antibiotics, and other supportive medications. Since there was not much improvement, an MRI of the brain was repeated after one week, which revealed SAH in the bilateral Sylvian fissures and cerebellar foliae, intraparenchymal hemorrhage in bilateral occipital lobes, with intraventricular hemorrhage [Figure 1]. His coagulation profile was normal and platelet counts were 1.3 lakhs/mm3 at the time of repeat imaging.
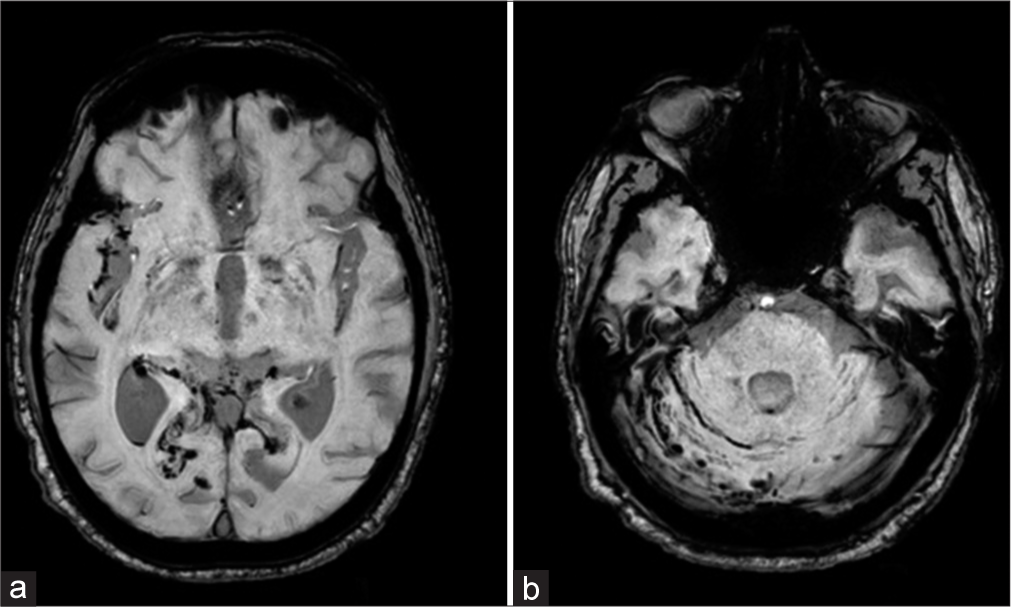
- Axial magnetic resonance imaging of the brain on susceptibility-weighted imaging shows (a) hemorrhage within the right Sylvian fissure, bilateral occipital lobes, the occipital horn of the right lateral ventricle, and (b) along the right cerebellar folia.
Follow-up computed tomogram (CT) scan [Figure 2] after a few days showed an increase in intraventricular and SAHs. Later, he developed paraparesis, and an MRI of the whole spine showed a hemorrhage extending into the subarachnoid space of the cervical spinal cord. Intradural hemorrhage and clots were noted within the thecal sac of the thoracic and lumbar spine, extending up to the filum terminale. Intramedullary hemorrhage was noted in the lower thoracic spinal cord from the T8 level to the conus with the expansion of the spinal cord [Figures 3 and 4].
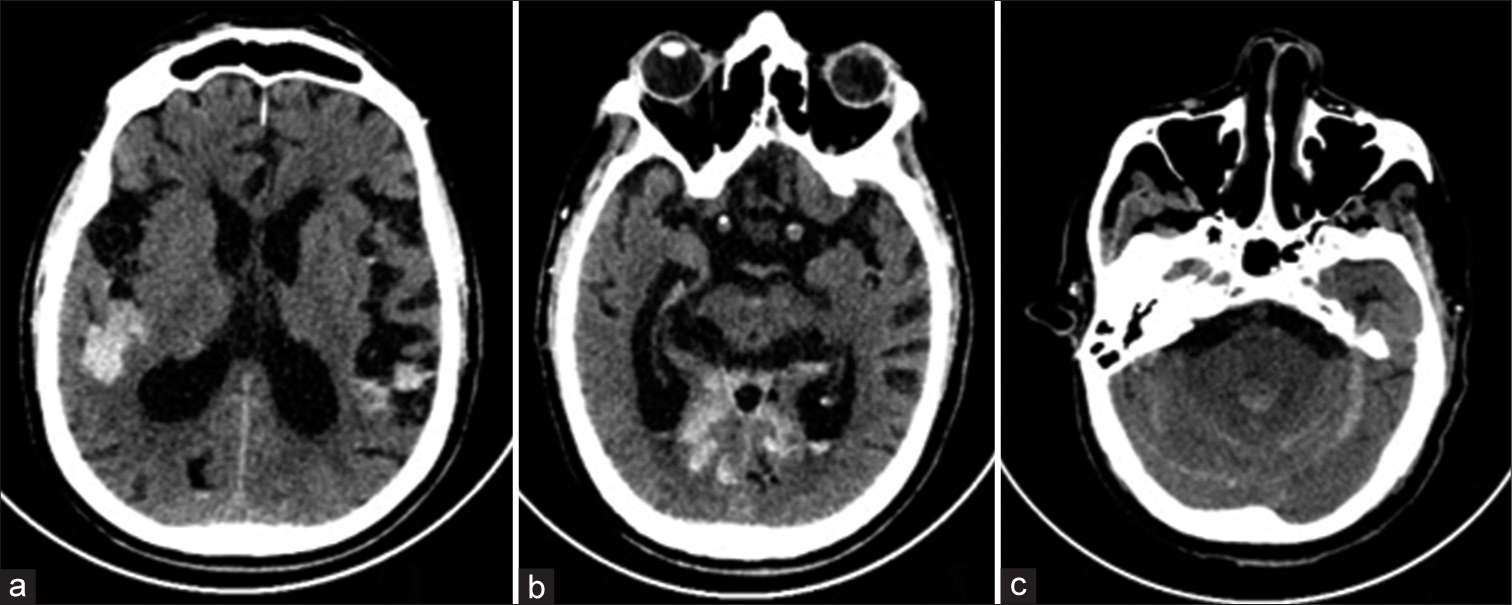
- Axial images of plain computed tomogram scan of the brain show subarachnoid hemorrhage in (a) bilateral Sylvian fissures and (c) cerebellar hemispheres. (b) Intraventricular hemorrhage in bilateral occipital horns and within the 4th ventricle.
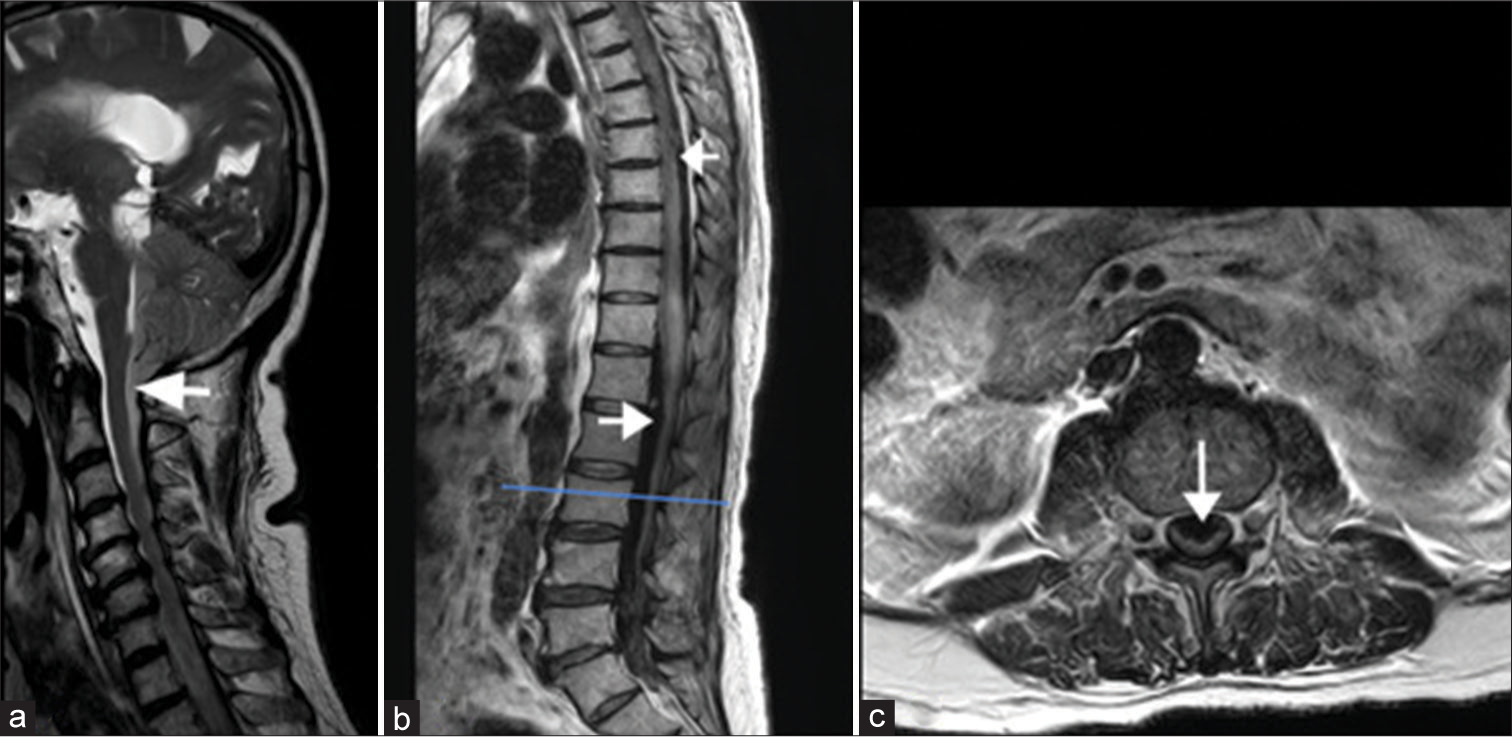
- T2-weighted sagittal image of the (a) cervical spine, (b) thoracolumbar spine, and (c) axial section of the lumbar spine: Subarachnoid hemorrhage (SAH) extending into the cervical spinal cord as shown by the arrow. SAH is also noted as hypointense signals anteriorly and posteriorly in thoracic and lumbar thecal sac indicated by arrows in (b). Axial T2WI (c) through the lower lumbar spine shows subarachnoid hematoma compressing the cauda equina nerve roots in (c).
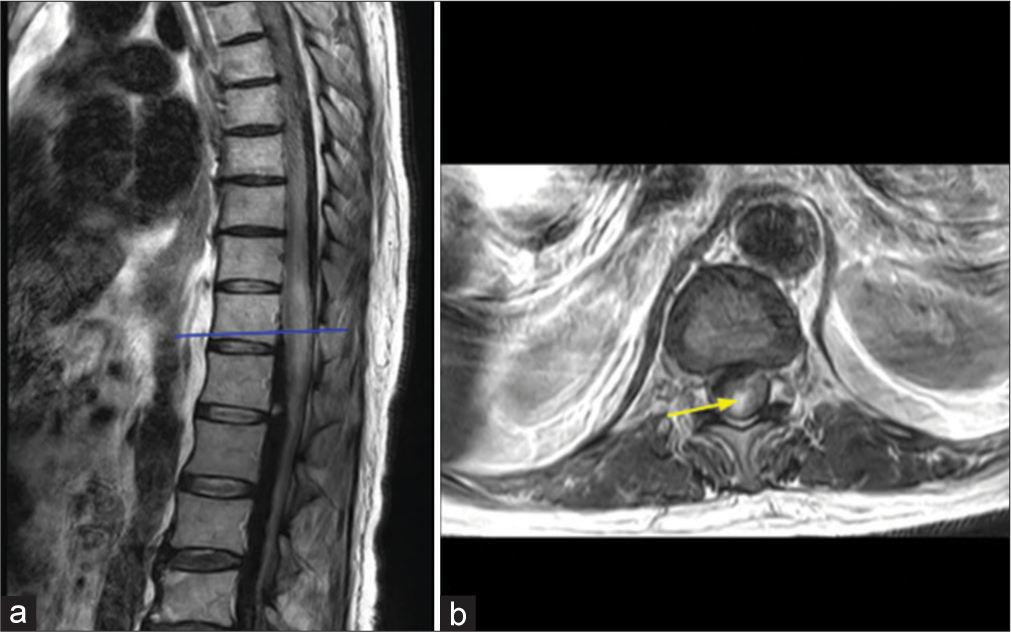
- T2-weighted (a) sagittal and (b) axial images at the level of the lower thoracic spinal cord show expansion of the spinal cord with intramedullary hemorrhage, as evidenced by (b) hyperintense signal shown by the arrow.
Therapeutic intervention
A continuous lumbar puncture drain was placed for 10 days. Cerebrospinal fluid (CSF) analysis showed neutrophilic pleocytosis with elevated protein and normal sugar levels. The CSF meningoencephalitis panel yielded negative results. He was treated with broad-spectrum antibiotics and put on non-invasive ventilator support. His blood counts normalized, but his neurological condition remained status quo with a Glasgow coma scale of E4V2M4.
Follow-up and outcome
A follow-up MRI [Figure 5] showed resolution of the SAH in the cervical spine, with residual subacute hematoma in the thoracolumbar levels, compressing the spinal cord and causing posterior displacement of the cauda equina nerve roots.
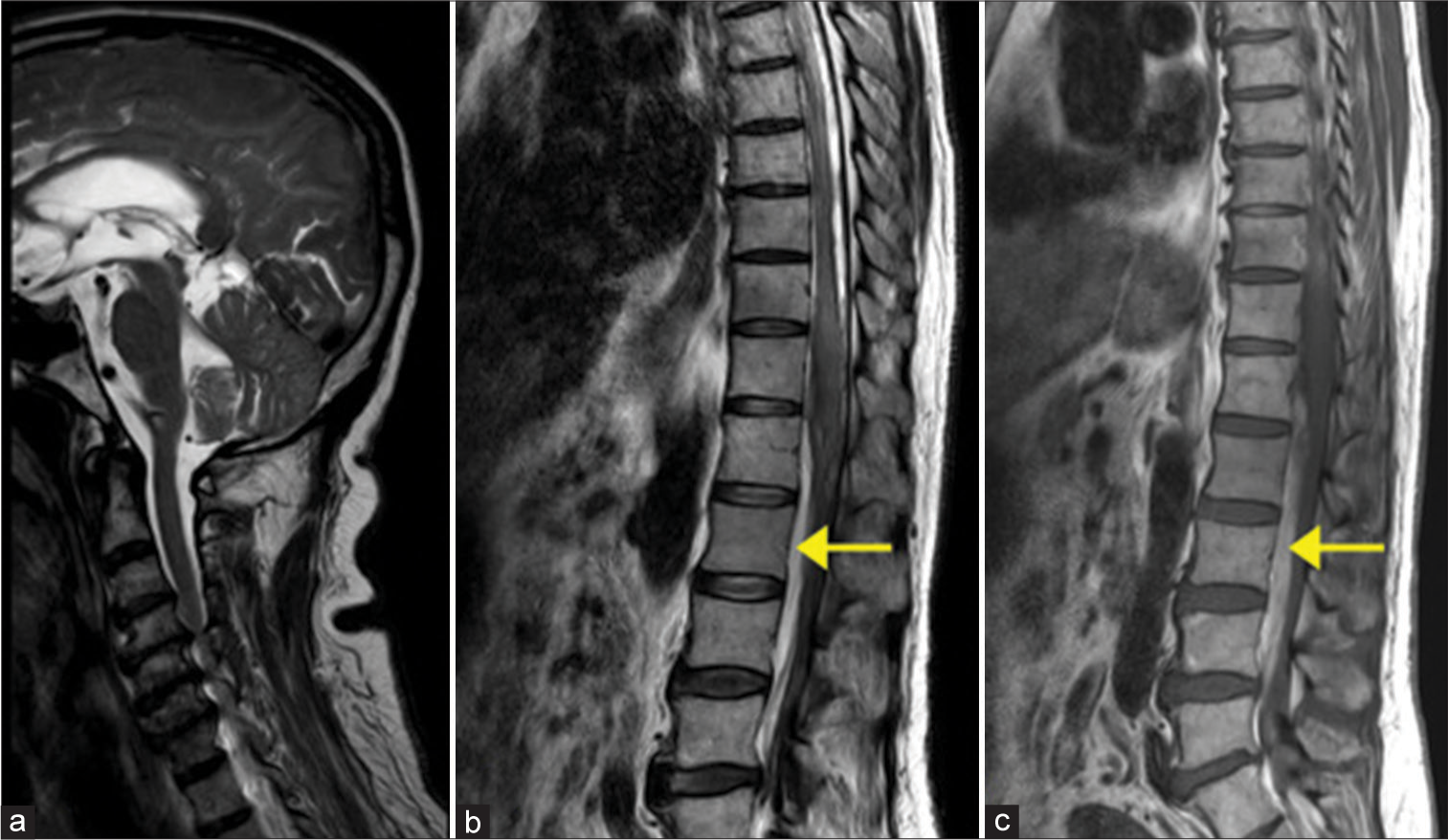
- T2-weighted sagittal images of the (a) cervical, (b) thoracolumbar spines, and (c) T1 weighted sagittal image of the thoracolumbar spine. (a) Resolution of the subarachnoid hemorrhage in the cervical spine. (b,c) Anterior residual subacute hematoma displacing the cauda equina nerve roots posteriorly is noted in the lumbar levels, appearing hyperintense on (b) T2-weighted images and (c) T1-weighted images, as shown by the arrows in (b) and (c).
Despite radiological improvement, the patient’s clinical condition demonstrated only slight progress on discharge from the hospital.
He was readmitted after a month with a urinary tract infection, a CT brain then showed resolution of intraparenchymal and SAHs. He was conscious, oriented with grade >3/5 power in both lower limbs and he was able to walk with support at three-month follow-up.
DISCUSSION
Dengue hemorrhagic fever is one of the most dreaded complications of dengue fever. Hemorrhagic complications occur due to thrombocytopenia, endothelial dysfunction, capillary leakage, coagulation abnormalities, and hepatic dysfunction, resulting in spontaneous hemorrhage from the skin and mucous membrane, the upper or lower gastrointestinal tract, and from venipuncture sites.[1] Neurological manifestations of dengue fever account for <1% of the total systemic complications.[2,3] The neurotrophic effects of the viruses are believed to cause encephalitis, meningitis, systemic complications, and post-infectious complications.[3,4] However, neurological complications have only been reported in 0.5–20% of cases.[3,4]
Intracranial hemorrhage is one of the rare complications seen in dengue, the pathogenesis of which is multifactorial, attributed to the complex interplay of vasculopathy, endothelial dysfunction, coagulopathy, platelet dysfunction, and thrombocytopenia.[5] Spontaneous intraspinal hemorrhage is extremely rare in dengue fever, and only a very few cases have been reported to date. Initially, Singh and Joseph reported a case of paraparesis caused by an epidural hematoma secondary to thrombocytopenia in dengue fever.[6] Kaur et al. reported a patient with cauda equina due to dengue-induced thrombocytopenia.[7] Few other cases of dengue also had associated intraspinal hemorrhage, one of them had cervical cord compression by an extramedullary hematoma causing quadriparesis[8] and spontaneous spinal SAH causing paraparesis in another,[9] while a third had normal platelet counts with quadriparesis as a result of hematomyelia due to platelet dysfunction.[10] A case of spontaneous spinal hemorrhage by Kaushik et al. with acute onset of paraplegia with bladder and bowel dysfunction was reported in dengue fever with severe thrombocytopenia.[11] In our case, the platelet levels had only dropped to 45,000/mm3 and we identified that the patient had developed intracranial and intraspinal hemorrhagic complications when platelet counts were more than 1 lakh/mm3.
The sudden deterioration of our patient’s sensorium might have resulted from a combination of dengue-related encephalitis, intracranial hemorrhage, and concomitant intraspinal hemorrhage causing cord compression. The intracranial and intraspinal hemorrhage could be attributable to endothelial dysfunction and due to changes in plasma levels of coagulation and fibrinolysis parameters. High levels of the permeability-enhancing and chemoattractant cytokines interleukin (IL)-6 and IL-8 were associated with dengue fever, and there is a significant association between IL-8 levels and thrombocytopenia. Intraspinal SAH has rarely presented with spinal cord compression due to the diluting and redistributing effects of CSF. However, subarachnoid hematoma formation can cause compression on the spinal cord as well as the cauda equina nerve roots. In the absence of neurological deficit, spinal SAH may resolve spontaneously.[12] Rapid diagnosis using MRI is critical in managing dengue-related intraspinal hemorrhage and for visualizing the extent of spinal involvement. The goal of surgery, when indicated, is to decompress the spinal cord or nerve roots, and stabilize the spine if necessary. Surgical options may include laminectomy for decompression and evacuation of hematoma, depending on the extent of the hemorrhage. Early diagnosis and spinal cord decompression could have resulted in a more favorable neurological outcome for our patient. However, this was not possible since the platelet count had to be raised to an optimal level to perform the decompression as well as to reduce further hemorrhage.
CONCLUSION
Dengue hemorrhagic fever can be further complicated by spontaneous intracranial or intraspinal hemorrhage and require a high index of suspicion, especially when the neurological condition worsens despite supportive treatment. This diagnosis should also be considered in any case of dengue fever with atypical neurological manifestations. Prompt recognition of symptoms and early imaging with MRI are crucial steps in the management of dengue-related intraspinal hemorrhage. In cases where surgery is indicated, timely intervention can significantly impact the prognosis and reduce the risk of permanent neurological deficits.
Availability of data
Original data is available and can be provided on request.
Ethical approval
The research/study approved by the Institutional Review Board at RENAI MEDICITY MULTI SUPER SPECIALTY HOSPITAL, KOCHI, number RMIEC/RIMS/535, dated November 6, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Dengue fever with diffuse cerebral hemorrhages, subdural hematoma and cranial diabetes insipidus. BMC Res Notes. 2016;9:265.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical manifestations of dengue. Trop Med Int Health. 2007;12:1087-95.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue infection: Not just fever-a rare presentation with literature review. J Pediatr Neurol. 2020;18:241-5.
- [CrossRef] [Google Scholar]
- Neurological complication of dengue infection. Neurol India. 2010;58:581-4.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous intracranial hemorrhage associated with dengue fever: An emerging concern for general physicians. J Family Med Prim Care. 2018;7:618-28.
- [CrossRef] [PubMed] [Google Scholar]
- Paraplegia in a patient with dengue. Neurol India. 2010;58:962.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue fever presenting as cauda equina syndrome. BMJ Case Rep. 2017;2017:bcr2017221251.
- [CrossRef] [PubMed] [Google Scholar]
- An atypical case of dengue haemorrhagic fever presenting as quadriparesis due to compressive myelopathy. BMJ Case Rep. 2011;2011:bcr1020103421.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous spinal subarachnoid haemorrhage: A rare complication of dengue fever. J Clin Health Sci. 2017;2:54-7.
- [CrossRef] [Google Scholar]
- A report of quadriparesis in dengue fever due to hematomyelia. Neurol India. 2019;67:530-1.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous spinal intradural hemorrhage in dengue fever: A case report. J Med Case Rep. 2022;16:213.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic spontaneous spinal subarachnoid hemorrhage. Spinal Cord. 2004;42:545-7.
- [CrossRef] [PubMed] [Google Scholar]







