Translate this page into:
Demography, Pattern of Care, and Survival in Patients with Xanthoastrocytoma: A Systematic Review and Individual Patient Data Analysis of 325 Cases
Rony Benson Department of Radiation Oncology, B.R.A. Institute-Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi India ronybenson@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives Xanthoastrocytoma (XA) is a low-grade glial tumor seen in young adults and there is lack of robust data on treatment of this rare tumor. In this systematic review and individual patient's data analysis, we aimed to look into the demography, pattern of care, survival outcomes, and prognostic factors in patients with both Grade II and III XA.
Methods A comprehensive search was conducted with the Medical Subject Heading terms: “Xanthoastrocytoma; Pleomorphic Xanthoastrocytoma; Anaplastic Xanthoastrocytoma; Xanthoastrocytoma AND treatment; and Anaplastic Xanthoastrocytoma AND survival” to find all possible publications.
Results A total of 325 individual patients from a total of 138 publications pertaining to XA were retrieved. Median age of the entire cohort was 19 years. About 56.1% of the patients underwent a gross total resection (GTR) and 31.4% underwent a subtotal resection. Nearly, 76.6% of the patients had a Grade II tumor and adjuvant radiation was delivered in 27.4% of the patients. Estimated 2- and 5-year progression-free survival (PFS) were 68.5 and 51.2%, respectively. Age, grade, and extent of surgery were significant factors affecting PFS. Estimated 2- and 5-year overall survival (OS) was 88.8 and 78%, respectively. The median OS for Grade II and Grade III tumors were 209 and 49 months, respectively. Age and extent of surgery were significant factors affecting OS.
Conclusion XA is a disease of young adults with favorable prognosis. Younger patients (<20 years), patients who undergo a GTR, and patients with a lower grade tumor have a better treatment outcome.
Keywords
meta-analysis
survival
xanthoastrocytoma
Introduction
Xanthoastrocytoma (XA) was first described by Kepes et al in 1979 as a low-grade glial tumor seen in young adults. XA usually presents with seizures and includes Grade II and Grade III tumors with distinct clinical behavior. Though most of the tumors are reported to arise from temporal lobe, these tumors have been reported to arise from any part of the central nervous system. Maximal safe surgical resection is often considered the cornerstone of therapy. Adjuvant therapy though advocated by many lacks consensus and merits a relook. However, the greatest limitation is the sporadic reporting of XA in the literature. In the absence of robust data, treatments are often based on local expertise and institutional protocol extrapolating data of other common glial tumors. Our earlier analysis was limited to patients of Grade II XA.1 In this systematic review and individual patient’s data analysis, we aimed to look into the demography, pattern of care, survival outcomes, and prognostic factors in patients with both Grade II and III XA.
Methods
Search Methodology
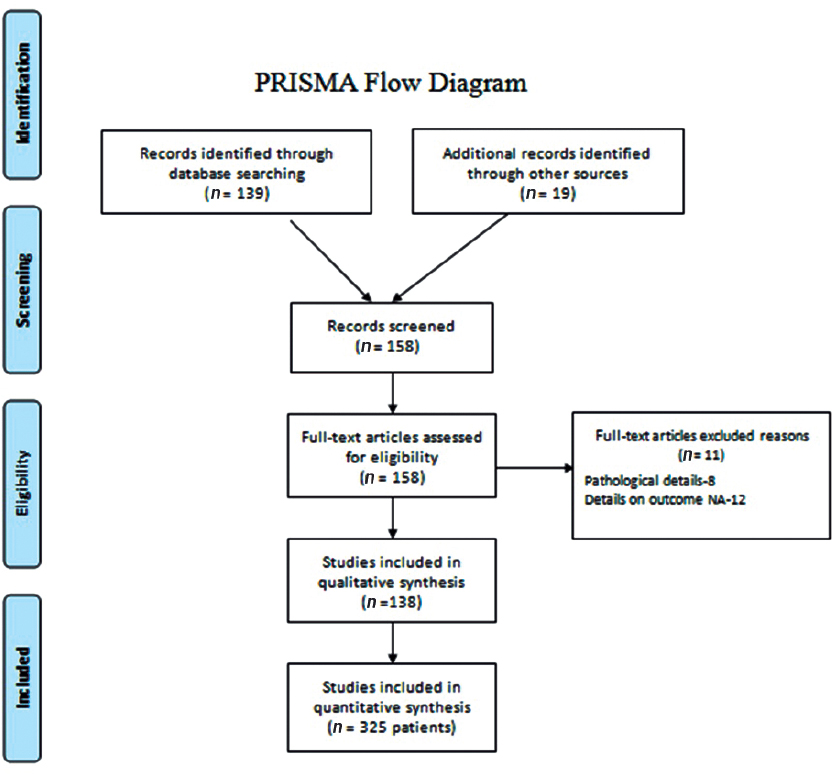
A comprehensive search was conducted in the PubMed, Google Scholar with the Medical Subject Heading terms: “Xanthoastrocytoma; Pleomorphic Xanthoastrocytoma; Anaplastic Xanthoastrocytoma; Xanthoastrocytoma AND treatment; and Anaplastic Xanthoastrocytoma AND survival” to find all possible publications. After retrieving the titles of such articles, we sorted out any unrelated articles. We retrieved full-length articles of those remaining to finalize articles for data extraction. In addition, we searched the references in those articles as well to fetch any article missing after the search. Thereafter, duplicates were removed, and the remaining articles were looked into detail. Patient data were extracted and entered in a predesigned excel chart with the headings of “age, gender, presenting complaints, type of surgery, radiation use, chemotherapy, recurrence, duration of progression-free interval, salvage treatment, death, and survival.” Articles that did not report treatment and outcome were excluded from the analysis. Once the data extraction was complete, it was rechecked by the individual authors to look for errors or duplication. A total of 138 articles were retrieved pertaining to XA with 325 patients.138 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart (Fig. 1) explains the data synthesis from the eligible studies.
-
Fig. 1 PRISMA flowchart showing summary of the search methodology and data collection for the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Fig. 1 PRISMA flowchart showing summary of the search methodology and data collection for the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Statistical Analysis
The data were analyzed and categorical variables were summarized by frequency and percentage and quantitative variables by the median and range. Progression-free survival (PFS) and overall survival (OS) were calculated from the date of diagnosis to the date of documented progression or death. Kaplan-Meier method was used to for survival analysis. Univariate analysis was performed using log-rank test, and Cox regression model was used for multivariate analysis. Factors with p-value < 0.1 were included in the multivariate analysis. A p-value < 0.05 was taken as significant. SPSS v.16 (SPSS Inc., Released 2007, SPSS for Windows, Version 16.0, Chicago, Illinois, United States) was used for all statistical analysis.
Results
We retrieved data of 325 individual patients from a total of 138 publications pertaining to XA. Median age of the entire cohort was 19 years (range: 0.9-84 years). More than four-fifth (82%) of the patients were diagnosed within the fourth decade. Of these 325 patients, incidence of XA was equally distributed among males and females with a ratio of 0.92:1 favoring females. Nearly half of the patients presented with features of raised intracranial pressure (47%) followed by seizure which was the presenting symptom in one-third of the patients. Out of 325 patients, 118 (36.3%) patients had tumor located in the temporal lobe only followed by multilobar (16%) disease and frontal lobe (9.8%). A total of 8 patients had tumor located in different parts of the spinal cord as well. Only two patients had leptomeningeal dissemination at diagnosis. Patient characteristics are summarized in Table 1.
|
Patient characteristics |
Number of patients (percentage)/(range) |
|---|---|
|
Abbreviations: CT, chemotherapy; RT, radiotherapy; TMZ, temozolomide. |
|
|
Age |
Median: 19 y (range: 0.9–84) |
|
Sex (n = 318) |
Male: 153 Female:165 Male:female ratio: 0.93:1 |
|
Presenting symptoms (n = 229) |
Seizure: 108 (47.2%) Headache: 76 (33.2%) Sensory symptoms: 26 (11.4%) Motor symptoms: 16 (6.9%) Hemorrhage: 3 (1.3%) |
|
Radiological feature (n = 61) |
Cystic: 44 (72.1%) Solid: 10 (16.4%) Solid-cystic: 7 (11.5%) |
|
Contrast enhancement: 35(57.3%) |
|
|
Ki-67 labeling index (n = 54) |
Median: 5.6% (range: 1–33.2%) |
|
Surgery (n = 287) |
Gross total or near total resection: 161 (56.1%) Subtotal resection or debulking: 90 (31.4%) Biopsy: 6 (2.1%) |
|
Grade (n = 218) |
Grade II: 167 (76.6%) Grade III: 51 (23.4%) |
|
BRAF mutation (N = 24) |
Yes: 14 (58.3%) No: 10 (41.7%) |
|
Radiation (N = 281) |
Adjuvant radiation: 77 (27.4%) No adjuvant radiation: 184 (65.5%) Palliative: 1 (0.3%) Salvage RT: 19 (6.8%) |
|
Chemotherapy (N = 249) |
Adjuvant: 37 (14.9%) (TMZ, n = 7) Not used: 208 (83.5%) Salvage-4 (1.6%) |
|
Salvage treatment (N = 76) |
Surgery: 20 (26.3%) Radiation: 13 (17.1%) Chemotherapy: 2 (2.6%) Surgery + RT: 13 (17.2%) CT + RT: 7 (9.2%) Surgery + CT: 3 (3.9%) Surgery + RT + CT: 18 (23.7%) |
Treatment
Surgical details were available in 287 cases. Of these, 161 (56.1%) patients underwent a gross total resection (GTR) and 90 (31.4%) patients underwent a subtotal resection. Histologic grade was available in 218 cases. Of these, 167 (76.6%) patients had a Grade II tumor and the remaining 23.4% patients had a Grade III tumor. Median Ki-67 was found to be 5.6% (range: 1-33.2%). In 14 patients (58.3%) out of 24 available cases, BRAF mutation was noted. Adjuvant radiation was delivered in 77 (27.4%) patients, whereas 1 patient was treated with palliative radiation and salvage radiation was used in 19 (6.8%) patients. In the available reports, all patients received local radiation alone. Adjuvant chemotherapy was used in 37 (14.9%) patients. Chemotherapy regimen varied widely; but in the recent report, temozolomide has been found to be the preferred choice (n = 7).
Survival Outcome
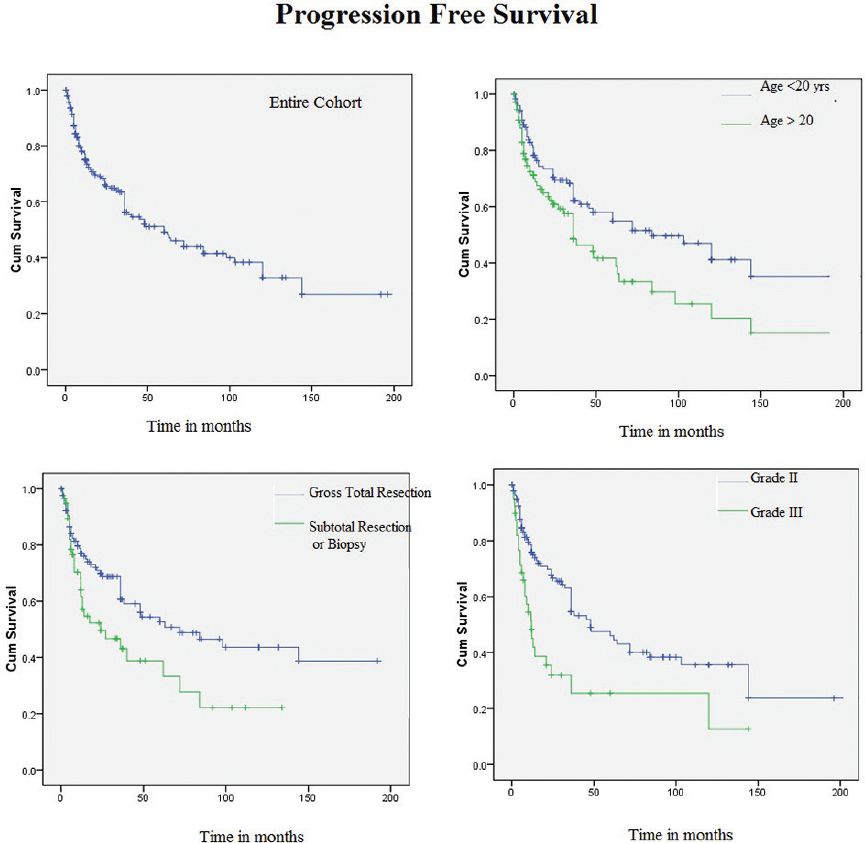
Estimated 2- and 5-year PFS were 68.5 and 51.2%, respectively. In univariate analysis, younger patients (≤20 years) found to have better PFS compared with elder patients (> 20 years) (hazard ratio [HR] 2.26 [95% confidence interval [CI]: 1.3-4.0], p = 0.006). Patients with a GTR had a significantly better PFS than those treated with a subtotal resection (STR) only (HR 2.19 [95% CI: 1.1-4.2], p = 0.019). PFS was found to be significantly better for those with a Grade II tumor compared with those with a Grade III tumor (HR 3.18 [95% CI: 1.6-6.4], p = 0.001) (Fig. 2). Age, grade, and extent of surgery continued to be significant in multivariate analysis with HR of 1.9 (95% CI: 1.2-8.2, p = 0.007), 2.0 (95% CI: 1.2-3.3, p = 0.005), and 1.9 (95% CI: 1.1-3.2, p = 0.018), respectively.
-
Fig. 2 Kaplan–Meier curves showing progression-free survival in patients with xanthoastrocytoma for entire cohort, with respect to age, nature of surgery, and grade of tumor.
Fig. 2 Kaplan–Meier curves showing progression-free survival in patients with xanthoastrocytoma for entire cohort, with respect to age, nature of surgery, and grade of tumor.
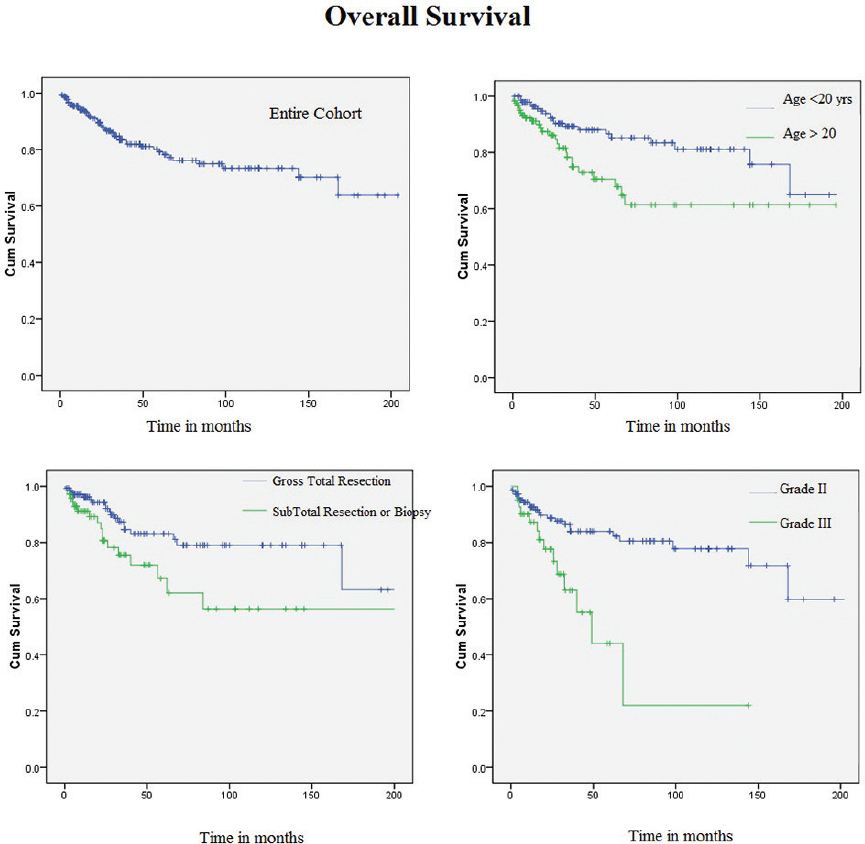
Estimated 2- and 5-year OS were 88.8 and 78%, respectively. In univariate analysis, younger patients (≤20 years) were found to have better OS compared with elder patients (> 20 years) (HR 1.58 [95% CI: 1.07-2.32], p = 0.019). Patients with a GTR had a significantly better OS than those treated with a STR only (HR 1.72 [95% CI: 1.1-2.71], p = 0.017). OS was found to be significantly better for those with a Grade II tumor compared with those with a Grade III tumor (HR 2.2 [95% CI: 1.4-3.6], p = 0.001) (Fig. 3). The median OS for Grade II and Grade III tumors were 209 and 49 months, respectively. The other factors such as sex, adjuvant chemotherapy, and adjuvant radiotherapy had no impact on OS. Age and extent of surgery continued to be significant in multivariate analysis with HR of 3.4 (95% CI: 1.4-8.4, p = 0.005) and 2.8 (95% CI: 1.3-6.3, p = 0.010), respectively. Grade of tumor lost its statistical significance in multivariate analysis.
-
Fig. 3 Kaplan–Meier curves showing overall survival in patients with xanthoastrocytoma for entire cohort, with respect to age, nature of surgery, and grade of tumor.
Fig. 3 Kaplan–Meier curves showing overall survival in patients with xanthoastrocytoma for entire cohort, with respect to age, nature of surgery, and grade of tumor.
Pattern of Recurrence and Salvage Treatment
Status of disease progression was documented in 275 patients. At a median follow-up of 32.4 months, 130 patients experienced disease progression. Most common pattern of recurrence was local but 9 (6.9%) patients had leptomeningeal dissemination. Details of salvage therapy were available in 76 (58.5%) patients. About 26.3% patients underwent a repeat surgery alone, and 17.1% patients received radiation as salvage treatment. However, a total of 54 (71.1%) patients underwent surgery followed by observation or other form of therapy as well. Of these 76 patients, 51 (67.1%) received radiation either alone or in combination with surgery and chemotherapy. Interestingly, only 5 of these 51 patients had received prior radiation. Salvage chemotherapy has been used in 30 (39.5%) patients. Chemotherapy regimen varied widely from temozolomide, bevacizumab, carmustine, lapatinib, irinotecan, flutamide, lomustine, cyclophosphamide, thiotepa, dabrafenib, etc. Interestingly, 4 patients were diagnosed with astrocytoma, glioblastoma, or anaplastic oligodendroglioma at recurrence.
Discussion
XA has long been considered an indolent tumor entity. Because of the rarity of this tumor, there is a paucity of data regarding its clinical behavior and dilemma regarding the optimum treatment of these patients. In the absence of level I evidence, the individual patient data analysis was formulated to describe the demography, pattern of care, and survival outcome for XA. The survival and prognostic variables for Grade II XA has already been described in an earlier article.1 Hence, the present article aimed to compare survival and prognostic factors for Grade III XA with that of Grade II tumors as well. We retrieved 138 publications and derived individual patient data of 325 odd patients for the purpose of the analysis. Interestingly, only 7 publications had a sample size of more than 10.
For the entire cohort of XA, median age was 19 years. Our previous analysis of Grade II XA highlights the median age of 20 years which is not different for the entire cohort as well. This clearly reflects that XA is predominantly a disease of the young adults. The present analysis revealed an impressive median OS of 209 months for Grade II tumors, while it was only 49 months for Grade III tumors. An earlier analysis of Grade II and Grade III XA reported similar median survival.139 Univariate analysis also pointed toward a better prognostic outcome for older patients (> 20 years) compared with the younger patients (< 20 years). Eighty-two percent of the cases are diagnosed up to the age of 40 years with isolated cases in different ages beyond 40 years.
A Surveillance, Epidemiology, and End Results (SEER) data analysis and our previous analysis highlight the importance of surgery in particular importance of achieving a GTR.139 Although, in multivariate analysis, extent of surgery was not significant in the SEER analysis, it was significant in the analysis for Grade II tumors. The most important limitation of interpreting the surgical extent is variation of surgeons, different centers with variable experience, and in long period ways of interpretation of completeness of surgery. In addition, surgical standards have improved over the last few years with modern techniques such as intraoperative magnetic resonance imaging (MRI), awake craniotomy, and postoperative MRI. With the limitations of all these variables, the present analysis revealed significant impact of a GTR both on PFS (HR 2.19, p = 0.019) and OS (HR 1.72, p = 0.017). These findings clearly highlight the importance of achieving a GTR in XA. The analysis also highlights the importance of referring such patients to a center with expertise for better management.
However, the impact of adjuvant radiation was not a significant factor influencing OS or PFS. However, point should also be made that many of the patients have received radiation in poor performance status or with a large tumor. Furthermore, a possibility of publication bias cannot be ignored. This also highlights adopting a risk adopted treatment approach for lower and higher grade tumors. Hence, a GTR should be optimum for a lower grade tumor but a higher grade tumor merits more aggressive therapy with adjuvant radiotherapy or a combination of radiotherapy and chemotherapy should be advocated.
Because of excellent treatment outcome, long-term squeal and neurocognitive function is very important for these patients and every effort should be made to assure a better quality of life for patients with XA. In addition, follow-up protocol should also be carefully designed. As local recurrence is predominant, a contrast-enhanced MRI of brain every 3 months for the first 3 years and thereafter 6 monthly for 2 years and then annually should be optimum.
In the recent years, great enthusiasm has been witnessed in exploring the molecular pattern of XA. Different reports have demonstrated nearly V600E BRAF mutation in nearly 70% patients which constitutively activates RAS/RAF/MEK/ ERK signaling pathway. Different BRAF inhibitors such as vemurafenib and dabrafenib have shown promising results in the management of recurrent XA. The present analysis revealed 58% BRAF mutation rate which makes it an interesting target for recurrent cases.
The analysis reveals many important facts about the rare tumor. We found significantly improved survival outcome for patients treated with a GTR than those with STR. Similarly, both PFS and OS favored patients with a Grade II tumor, younger age. However, the impact of adjuvant therapy was not clearly beneficial. Note should be made that nearly 47.7% patients experience disease progression at a median follow-up of nearly 3 years. Hence, risk stratification should be done and adjuvant radiation and chemotherapy may be advocated for high-risk patients.
This analysis has few limitations as well. As the individual patient data has been extracted from publication over a long period of time, a temporal bias is paramount importance. In this time frame, diagnostic criteria, surgical skill, and treatment approach have changed which may have definite impact on the quality of report. In addition, the publications included in the analysis are retrospective which also add to different types of bias. Because of inhomogeneity in reporting the cases, all relevant data pertaining to each case were not retrieved. This also forced us to conduct analysis on available fraction of data for fewer parameters. The use of individual patient characteristics for analysis may be considered as one of the merits of this work.
Conclusion
XA is a disease of young adults with favorable prognosis. Younger patients (< 20 years), patients who undergo a GTR, and patients with a lower grade tumor have a better treatment outcome. The role of adjuvant therapy is debatable. However, in Grade III and incompletely resected tumors, adjuvant radiation of a combination of both radiotherapy and chemotherapy should be delivered. Whole genome sequencing should be performed to identify patients with different clinical behavior and treat accordingly.
Conflict of Interest
None declared.
Funding None.
References
- Grade II pleomorphic xanthoastrocytoma; a meta-analysis of data from previously reported 167 cases. J Clin Neurosci. 2018;54:57-62.
- [Google Scholar]
- Cerebellar pleomorphic xanthoastrocytoma: case report and literature review. Surg Neurol. 2007;68(1):89-94. discussion 94-95
- [Google Scholar]
- Cerebral pleomorphic xanthoastrocytoma associated with NF1: an updated review with a rare atypical case from Africa. Neurosurg Rev. 2012;35(3):313-319. discussion 319
- [Google Scholar]
- Composite pleomorphic xanthoastrocytoma-epithelioid glioneuronal tumor with BRAF V600E mutation - report of three cases. Clin Neuropathol. 2014;33(2):112-121.
- [Google Scholar]
- Epithelioid glioblastomas and anaplastic epithelioid pleomorphic xanthoastrocytomas - same entity or first cousins? Brain Pathol. 2016;26(2):215-223.
- [Google Scholar]
- Malignant progression of a pleomorphic xanthoastrocytoma in a child. Neuropediatrics. 2010;41(2):69-71.
- [Google Scholar]
- Intrasellar pleomorphic xanthoastrocytoma: case report. Neurosurgery. 2002;51(4):1079-1082. discussion 1082
- [Google Scholar]
- A case of anaplastic pleomorphic xanthoastrocytoma presenting with tumor bleeding and cerebrospinal fluid dissemination. Brain Tumor Pathol. 2006;23(1):55-63.
- [Google Scholar]
- Anaplastic pleomorphic xanthoastrocytoma with spinal leptomeningeal spread at the time of diagnosis in an adult. J Clin Neurosci. 2015;22(8):1370-1373.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with malignant transformation and multiple recurrences in an Iranian girl. BMJ Case Rep.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: report of a case diagnosed by intraoperative cytopathological examination. Diagn Cytopathol. 2001;24(2):120-122.
- [Google Scholar]
- Dabrafenib in BRAFV600-mutated anaplastic pleomorphic xanthoastrocytoma. CNS Oncol. 2017;6(1):5-9.
- [Google Scholar]
- Atypical teratoid/rhabdoid tumor arising in the setting of a pleomorphic xanthoastrocytoma. J Neurooncol. 2007;84(2):217-222.
- [Google Scholar]
- Malignant transformation in pleomorphic xanthoastrocytoma-a report of two cases. Br. J Neurosurg. 1999;13(5):516-519.
- [Google Scholar]
- Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol. 2013;114(2):237-240.
- [Google Scholar]
- Pediatric cerebellar pleomorphic xanthoastrocytoma with anaplastic features: a case of long-term survival after multimodality therapy. Childs Nerv Syst. 2006;22(6):609-613.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with malignant progression. Surg Neurol. 1998;50(4):385-386.
- [Google Scholar]
- Primary anaplastic pleomorphic xanthoastrocytoma in adults. Case report and review of literature. Int J Surg Case Rep. 2016;27:183-188.
- [Google Scholar]
- Combined pleomorphic xanthoastrocytoma-ganglioglioma with BRAF V600E mutation: case report. J Neurosurg Pediatr. 2016;18(1):53-57.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma of the spinal cord: case report and literature review. Clin Neuropathol. 2014;33(3):190-196.
- [Google Scholar]
- Malignant transformation of pleomorphic xanthoastrocytoma: case report. Arq Neuropsiquiatr. 2003;61(1):104-106.
- [Google Scholar]
- Distinctive pleomorphic xanthoastrocytoma-like tumor with exclusive abortive or aberrant neuronal differentiation and repeated recurrence-case report. Neurol Med Chir (Tokyo). 2002;42(9):399-405.
- [Google Scholar]
- Positron emission tomography in three children with pleomorphic xanthoastrocytoma. J Child Neurol. 2002;17(7):522-527.
- [Google Scholar]
- Combined pleomorphic xanthoastrocytoma-ganglioglioma of the cerebellum. Arch Pathol Lab Med. 2000;124(11):1707-1709.
- [Google Scholar]
- Metastatic pleomorphic xanthoastrocytoma in the scalp. J Clin Neurosci. 2011;18(4):565-567.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection. Neuro-oncol. 2001;3(3):184-192.
- [Google Scholar]
- A 28-year-old man with headache, visual and aphasic speech disturbances. Brain Pathol. 2009;19(1):163-166.
- [Google Scholar]
- Intraventricular pleomorphic xanthoastrocytoma with anaplastic features. Neuropathology. 2010;30(4):443-448.
- [Google Scholar]
- Temporal lobe tumor demonstrating ganglioglioma and pleomorphic xanthoastrocytoma components. Case report. J Neurosurg. 1992;77(1):143-147.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with anaplastic features presenting without GFAP immunoreactivity: implications for differential diagnosis. Neuropathology. 2005;25(3):241-246.
- [Google Scholar]
- Delayed occurrence of cerebellar pleomorphic xanthoastrocytoma after supratentorial pleomorphic xanthoastrocytoma removal. Case report. J Neurosurg. 1995;82(1):116-118.
- [Google Scholar]
- Experience with gliomas in patients presenting with a chronic seizure disorder. Clin Neurosurg. 1986;33:15-42.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: magnetic resonance imaging findings in a series of cases with histopathological confirmation. Arq Neuropsiquiatr. 2013;71(1):35-39.
- [Google Scholar]
- Melanosomal melanin pigment in pleomorphic xanthoastrocytoma, evidence for neuronal-glial origin: a case report with review of the literature. Neuropathology. 2017;37(2):116-121.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: an atypical astrocytoma. J Pak Med Assoc. 2012;62(2):175-177.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma and oligodendroglioma: collision of 2 morphologically and genetically distinct anaplastic components. J Neurosurg. 2011;114(6):1648-1653.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma in the spinal cord. Case report. J Neurosurg. 1994;80(3):564-569.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: a comparative pathological study between conventional and anaplastic types. Histopathology. 2008;52(2):183-193.
- [Google Scholar]
- BRAF-mutated pleomorphic xanthoastrocytoma of the spinal cord with eventual anaplastic transformation. World Neurosurg. 2017;98:871.e9-871.e15.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma and moyamoya disease in a patient with neurofibromatosis type 1 - case report. Neurol Med Chir (Tokyo). 2011;51(4):310-314.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: a developmental glioneuronal tumor with prominent glioproliferative changes. J Neurooncol. 2004;66:17-27. 1-2
- [Google Scholar]
- Epithelial properties of pleomorphic xanthoastrocytomas determined in ultrastructural and immunohistochemical studies. Acta Neuropathol. 1987;74(2):142-150.
- [Google Scholar]
- Unique presentation of pleomorphic xanthoastrocytoma as a lytic skull lesion in an eight-year-old girl. Pediatr Neurosurg. 2002;37(5):254-257.
- [Google Scholar]
- Atypical teratoid/rhabdoid tumor arising in pleomorphic xanthoastrocytoma: a case report. Neuropathology. 2014;34(4):398-405.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma arising from the suprasellar region: a report of two cases. J Clin Neurosci. 2016;33:228-231.
- [Google Scholar]
- Angiomatous pleomorphic xanthoastrocytoma: a case report and literature review. Diagn Pathol. 2016;11(1):73.
- [Google Scholar]
- Cytologic features of pleomorphic xanthoastrocytoma, WHO grade II. A comparative study with glioblastoma. Diagn Cytopathol. 2017;45(4):339-344.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma arising from olfactory groove: a rare location for a rare tumor. Pediatr Neurosurg. 2013;49(5):292-296.
- [Google Scholar]
- Case of pleomorphic xanthoastrocytoma with anaplastic features in the pineal gland. Brain Tumor Pathol. 2013;30(4):242-246.
- [Google Scholar]
- Histopathological features of recurrent pleomorphic xanthoastrocytomas: further corroboration of the glial nature of this neoplasm. A study of 3 cases. Acta Neuropathol. 1989;78(6):585-593.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma associated with long-standing Taylor-type IIB-focal cortical dysplasia in an adult. Pathol Res Pract. 2009;205(2):113-117.
- [Google Scholar]
- Long-term control of disseminated pleomorphic xanthoastrocytoma with anaplastic features by means of stereotactic irradiation. Neuro-oncol. 2009;11(4):446-451.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma and desmoplastic infantile ganglioglioma-have these neoplasms a common origin? Folia Neuropathol. 1994;32(4):237-239.
- [Google Scholar]
- Pigmented astrocytoma with suprasellar location: case report and literature review. Acta Neuropathol. 2004;108(5):461-466.
- [Google Scholar]
- Cerebellar hemisphere, an uncommon location for pleomorphic xanthoastrocytoma and lipidized glioblastoma multiformis. Neurol India. 2003;51(2):246-247.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma of the cerebellopontine angle in a child. Childs Nerv Syst. 2006;22(11):1479-1482.
- [Google Scholar]
- Association of pleomorphic xanthoastrocytoma with cortical dysplasia and neuronal tumors. A report of three cases. Cancer. 1996;78(12):2551-2563.
- [Google Scholar]
- Cerebrospinal fluid cytologic findings of a pleomorphic xanthoastrocytoma: a case report. Acta Cytol. 2010;54(05):871-874.
- [Google Scholar]
- Successful treatment of a progressive BRAF V600E-mutated anaplastic pleomorphic xanthoastrocytoma with vemurafenib monotherapy. J Clin Oncol. 2016;34(10):e87-e89.
- [Google Scholar]
- Fatal pleomorphic xanthoastrocytoma with meningeal gliomatosis. Histopathology. 1998;32(4):375-378.
- [Google Scholar]
- Prognostic factors and therapeutic outcomes in 22 patients with pleomorphic xanthoastrocytoma. J Korean Neurosurg Soc. 2013;53(5):281-287.
- [Google Scholar]
- Cerebellar pleomorphic xanthoastrocytoma in an infant. Pathol Int. 1999;49(9):811-815.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma as a component of a cerebellar ganglioglioma: case report. Neurosurgery. 1992;31(2):353-355.
- [Google Scholar]
- Cerebral anaplastic pleomorphic xanthoastrocytoma with meningeal dissemination at first presentation. Childs Nerv Syst. 2004;20(2):119-122.
- [Google Scholar]
- Increased mitotic activity as a negative prognostic indicator in pleomorphic xanthoastrocytoma. Case report. J Neurosurg. 1993;79(5):761-768.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma in a case of tuberous sclerosis. J Neurosci Rural Pract. 2014;5(3):258-260.
- [Google Scholar]
- Malignant progression in pleomorphic xanthoastrocytoma: personal experience and review of the literature. J Neurol Sci. 2007;252(2):144-153.
- [Google Scholar]
- MRI of pleomorphic xanthoastrocytoma: case report. Neuroradiology. 1994;36(6):446-447.
- [Google Scholar]
- Synchronous multicentric pleomorphic xanthoastrocytoma: case report. Neurosurgery. 2005;57(1):E191.
- [Google Scholar]
- Intraventricular pleomorphic xanthoastrocytoma: a case report. Turk Neurosurg. 2014;24(6):987-991.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with predominantly exophytic growth: case report. Surg Neurol. 2001;56(5):330-332.
- [Google Scholar]
- Primary multicentric anaplastic pleomorphic xanthoastrocytoma with atypical features. J Clin Neurosci. 2013;20(11):1605-1608.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma of childhood: MR imaging and diffusion MR imaging features. AJNR Am J Neuroradiol. 2014;35(11):2192-2196.
- [Google Scholar]
- The coexistence of pleomorphic xanthoastrocytoma and arteriovenous malformation. A case report. Folia Neuropathol. 2013;51(3):269-274.
- [Google Scholar]
- Cerebellar pleomorphic xanthoastrocytoma in a patient with neurofibromatosis type 1. Neuroradiology. 2004;46(10):825-829.
- [Google Scholar]
- Malignant transformation of pleomorphic xanthoastrocytoma. Acta Neurochir (Wien). 2006;148(1):67-71.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma of the spinal cord. Case report. J Neurosurg Spine. 2006;5(1):72-75.
- [Google Scholar]
- Molecular genetic analysis of anaplastic pleomorphic xanthoastrocytoma. Asian J Surg. 2003;26(2):120-125.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma in two siblings with neurofibromatosis type 1 (NF-1) Clin Neuropathol. . 2012;31(1):54-56.
- [Google Scholar]
- Spinal imaging in intracranial primary pleomorphic xanthoastrocytoma with anaplastic features. J Clin Neurosci. 2012;19(9):1299-1301.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma in elderly patients may portend a poor prognosis. J Clin Neurosci. 2008;15(4):476-478.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with anaplastic features: a case report. Indian J Pathol Microbiol. 2014;57(1):101-104.
- [Google Scholar]
- Pleomorphic xanthoastrocytomas: institutional experience of 18 patients. J Clin Neurosci. 2014;21(10):1767-1772.
- [Google Scholar]
- Primary anaplastic pleomorphic xanthoastrocytoma with widespread neuroaxis dissemination at diagnosis-a pediatric case report and review of the literature. J Neurooncol. 2009;94(3):431-437.
- [Google Scholar]
- Non-anaplastic pleomorphic xanthoastrocytoma with neuroradiological evidences of leptomeningeal dissemination. Childs Nerv Syst. 2006;22(6):614-618.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with anaplastic features: a rare case report and review of literature with reference to current management. Asian J Neurosurg. 2016;11(3):319.
- [Google Scholar]
- Molecular genetic alterations in pleomorphic xanthoastrocytoma. Acta Neuropathol. 1996;91(3):293-297.
- [Google Scholar]
- Composite pleomorphic xanthoastrocytoma and ganglioglioma: report of four cases and review of the literature. Am J Surg Pathol. 1997;21(7):763-771.
- [Google Scholar]
- Combined oligodendroglioma/pleomorphic xanthoastrocytoma: a probable collision tumor: case report. Neurosurgery. 2001;48(6):1358-1361.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma arising in neurofibromatosis Type 1. Clin Neuropathol. 2012;31(3):152-154.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma derived from glioneuronal malformation in a child with intractable epilepsy. J Child Neurol. 2000;15(4):270-272.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma in children and adolescents. Pediatr Blood. Cancer. 2010;55(2):290-294.
- [Google Scholar]
- Unusual recurrence of pleomorphic xanthoastrocytoma. Br. J Neurosurg. 2006;20(6):433-434.
- [Google Scholar]
- Angiomatous pleomorphic xanthoastrocytoma as a component of ganglioglioma. Ann Saudi Med. 1999;19(1):48-51.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma of the cerebellum. Clin Neuropathol. 2000;19(5):238-242.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with anaplastic features: retrospective case series. World Neurosurg. 2016;95:368-374.
- [Google Scholar]
- Multicentric pleomorphic xanthoastrocytoma in a patient with neurofibromatosis type 1. Case report and review of the literature. J Neurosurg. 2005;102(2):376-381.
- [Google Scholar]
- Cytogenetic findings in a pleomorphic xanthoastrocytoma. Cancer Genet Cytogenet. 1991;55(2):225-230.
- [Google Scholar]
- Atrial fibrillation as an uncommon presentation in a large pleomorphic xanthoastrocytoma. Childs Nerv Syst. 2012;28(3):475-479.
- [Google Scholar]
- Does the occurrence of pleomorphic xanthoastrocytoma in the elderly carries a poor prognosis: a case report and review of literature. Asian J Neurosurg. 2014;9(4):237.
- [Google Scholar]
- Pigmented pleomorphic xanthoastrocytoma: report of a rare case with review of the literature. Arch Pathol Lab Med. 2001;125(6):808-811.
- [Google Scholar]
- Spinal pleomorphic xanthoastrocytoma. Case report [in Spanish] Neurocirugia (Astur). 2010;21(5):390-395.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma of the pineal region. J Clin Neurosci. 2010;17(11):1439-1441.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: report of two cases. Neurosurgery. 1988;22(2):422-427.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma as a component of a temporal lobe cystic ganglioglioma: a case report. Brain Tumor Pathol. 2009;26(1):31-36.
- [Google Scholar]
- Clinicopathological study of pleomorphic xanthoastrocytoma: correlation between histological features and prognosis. Pathol Int. 2000;50(9):703-708.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma-a clinicopathological study. Indian J Pathol Microbiol. 2000;43(3):357-361.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with anaplastic features in the Tectal region in a young adult patient: a case report. World Neurosurg. 2016;94:580.e11-580.e15.
- [Google Scholar]
- Cerebellar pleomorphic xanthoastrocytoma in a patient with neurofibromatosis type 1: a case report and literature review. Int J Clin Exp Pathol. 2015;8(6):7570-7574.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma:report of two cases and review of the prognostic factors. J Clin Neurosci. 2004;11(2):203-207.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: report of six cases with special consideration of diagnostic and therapeutic pitfalls. Surg Neurol. 1997;47(2):162-169.
- [Google Scholar]
- Anaplastic pleomorphic xanthoastrocytoma with a component of anaplastic astrocytoma presenting as skull base tumor followed by downward extracranial extension. Case report. Neurol Med Chir (Tokyo). 2010;50(12):1108-1112.
- [Google Scholar]
- Evaluation of pleomorphic xanthoastrocytoma by use of positron emission tomography with. AJNR Am J Neuroradiol. 2001;22(2):311-313.
- [Google Scholar]
- Primary meningeal pleomorphic xanthoastrocytoma with anaplastic features: a report of 2 cases, one with BRAF(V600E) mutation and clinical response to the BRAF inhibitor dabrafenib. J Neuropathol. Exp Neurol. 2015;74(10):960-969.
- [Google Scholar]
- Glycogen-rich pleomorphic xanthoastrocytoma with clear-cell features: confirmatory report of a rare variant with implications for differential diagnosis. Pathol Res Pract. 2011;207(4):256-261.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma with gangliogliomatous component. Pathol Res Pract. 1997;193(9):617-621.
- [Google Scholar]
- Clinical, radiological, and therapeutic features of pleomorphic xanthoastrocytoma: report of three patients and review of the literature. J Neurol Neurosurg Psychiatry. 1996;60(6):690-692.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: report of two cases with unconventional clinical presentations. Clin Neuropathol. 2014;33(6):380-387.
- [Google Scholar]
- Malignant potential of pleomorphic xanthoastrocytoma. J Clin Neurosci. 2012;19(1):12-20.
- [Google Scholar]
- Temporal lobe pleomorphic xanthoastrocytoma and chronic epilepsy: long-term surgical outcomes. Clin Neurol Neurosurg. 2011;113(10):918-922.
- [Google Scholar]
- Cerebellar pleomorphic xanthoastrocytoma: case report. Clin Neuropathol. 2000;19:238-242.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma. Report of four cases. J Neurosurg. 1989;70(3):463-468.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma presenting with life-threatening hemorrhage in a child. J Neurosurg Pediatr. 2009;3(2):157-159.
- [Google Scholar]
- Pigmented pleomorphic xanthoastrocytoma: a rare variant and literature review. Neuropathology. 2011;31(1):88-92.
- [Google Scholar]
- Glioblastoma multiforme versus pleomorphic xanthoastrocytoma with anaplastic features in the pathological diagnosis: a case report. Diagn Pathol. 2016;11(1):65.
- [Google Scholar]
- Possible differentiation of cerebral glioblastoma into pleomorphic xanthoastrocytoma: an unusual case in an infant. J Neurosurg Pediatr. 2012;9(5):517-523.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma in the lateral ventricle with extensive subarachnoid dissemination: report of a case and review of the literature. Chin Med J (Engl). 2012;125(2):396-399.
- [Google Scholar]
- Composite pleomorphic xanthoastrocytoma-ganglioglioma presenting as a suprasellar mass: case report. Neurosurgery. 2003;52(6):1465-1468. discussion 1468ߝ1469
- [Google Scholar]
- Genetic imbalances in pleomorphic xanthoastrocytoma detected by comparative genomic hybridization and literature review. Cancer Genet Cytogenet. 2002;132(1):14-19.
- [Google Scholar]
- Massive intracranial hemorrhage associated with pleomorphic xanthoastrocytoma-case report. Neurol Med Chir (Tokyo). 2010;50(3):220-223.
- [Google Scholar]
- Pleomorphic xanthoastrocytoma: MR imaging findings in 19 patients. Acta Radiol. 2011;52(2):223-228.
- [Google Scholar]
- Prevalence of mutated TP53 on cDNA (but not on DNA template) in pleomorphic xanthoastrocytoma with positive TP53 immunohistochemistry. Cancer Genet Cytogenet. 2009;193(2):93-97.
- [Google Scholar]
- Spinal pleomorphic xanthoastrocytoma companied with periventricular tumor. Int J Clin Exp Pathol. 2015;8(1):1036-1040.
- [Google Scholar]
- Molecular genetic and proteomic analysis of synchronous malignant gliomas. Neurology. 2004;62(12):2316-2319.
- [Google Scholar]
- Patterns of care and outcomes of patients with pleomorphic xanthoastrocytoma: a SEER analysis. J Neurooncol. 2012;110(1):99-104.
- [Google Scholar]






