Translate this page into:
Correlation between plasma total nitric oxide levels and cerebral vasospasm and clinical outcome in patients with aneurysmal subarachnoid hemorrhage in Indian population
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Cerebral vasospasm remains a major cause of morbidity and mortality in patients with aneurysmal subarachnoid hemorrhage (aSAH). Reduced bioavailability of nitric oxide has been associated with the development of cerebral vasospasm after aSAH. Such data is not available in Indian population.
Aims:
The objective of the study was to measure the plasma total nitric oxide (nitrite and nitrate-NOx) level in aSAH patients and healthy controls treated at a tertiary hospital in India and to investigate a possible association between plasma total nitric oxide level and cerebral vasospasm and clinical outcome following treatment in patients with aSAH.
Settings and Design:
A case-control study of aSAH patients was conducted. Plasma total NOx levels were estimated in aSAH patients with and without vasospasm and compared the results with NOx levels in healthy individuals.
Materials and Methods:
aSAH in patients was diagnosed on the basis of clinical and neuro-imaging findings. Plasma total NOx levels in different subject groups were determined by Griess assay.
Results:
Plasma total NOx level was found to be significantly decreased in patients with aSAH when compared to controls. Plasma total NOx level in the poor-grade SAH group was lower than that in the good-grade SAH group. Plasma total NOx level further reduced in patients with angiographic (P < 0.05) and clinical vasospasm.
Conclusions:
Reduced plasma NOx level is seen in aSAH patients as compared to normal individuals. In aSAH patients reduced levels are associated with increased incidence of cerebral vasospasm and poor outcome. Plasma total NOx level could be used as a candidate biomarker for predicting vasospasm and outcome for this pathology.
Keywords
Aneurysmal subarachnoid hemorrhage
cerebral vasospasm
nitric oxide
poor outcome
Introduction
Cerebral vasospasm is one of the dreaded complications following aneurysmal subarachnoid hemorrhage (aSAH) leading to delayed cerebral ischemia and permanent neurological deficits or even death.[1]
Unfortunately, the molecular mechanisms behind the development of cerebral vasospasm remain unclear despite extensive worldwide research and studies. The etiology and patho-physiology of development of vasospasm seem to be complex and multi-factorial.[2] Cellular and inflammatory processes are probably involved in the development of vasoconstriction.[3] Some studies hypothesize that increased levels of vasoconstrictors like endothelins, thromboxane A2 (TXA2) and platelet-derived growth factor (PDGF) promote vasospasm.[456789] On the other hand, there are reports which say vasospasm is due to reduced levels of vasodilators such as nitric oxide (NO) and prostacyclins.[481011] The exact pathway involved in the development of vasospasm is yet to be deduced.
NO is an important gaseous signaling molecule with many physiological roles. It serves as an endogenous vasodilator, platelet inhibitor, antioxidant and regulator of vascular endothelium by sustaining its anticoagulant and anti thrombogenic properties in different tissues.[12] NO is synthesized from l-arginine via Ca2+ -dependent family of enzymes. There are three isoforms of nitric oxide synthases (NOS), endothelial NOS (eNOS or NOS-3), neuronal NOS (nNOS or NOS-1) which are Ca2+ -dependent and Ca2+ -independent inducible NOS (iNOS-NOS-2). In the brain, vascular tone is regulated by NO produced by the endothelial NOS (eNOS) in the intima and neuronal NOS (nNOS) in the adventitia of cerebral vessels. iNOS is produced by glial cell.[13]
Several experimental and clinical studies have shown vasospasm attenuation by the administration of NO donors[4101415] or precursor of NO synthesis.[16] The plasma total NOx level (nitrites and nitrates) in aSAH patients with and without vasospasm were investigated and correlated for significance with clinical outcome and compared with plasma total NOx level in healthy individuals.
Materials and Methods
Subjects
This case-control study (prospective) was conducted at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India, a tertiary care center for neuropsychiatric disorders. The proposal was cleared by Institutional human ethical committee. Patient group consisted of patients, aged between 18 and 80 years, admitted between April 2011 and May 2012 with spontaneous aSAH. aSAH was diagnosed on the basis of clinical and neuro-imaging findings (computed tomography (CT), magnetic resonance angiography (MRA) and digital subtraction angiography (DSA)). Clinical outcome was assessd by Glasgow outcome scale (GOS-1 dead, GOS-2 vegetative state, GOS-3 severely disabled, GOS-4 moderately disabled and GOS-5 good recovery) at the time of discharge. The clinical and demographic data were entered into a standard proforma.
Control group comprised of healthy individuals, age-matched, clinically normal, who had no prior history of cerebral vascular disease, recruited from the institute staff and other volunteers were studied. An informed consent was obtained from all the patients and healthy volunteers willing to participate in the study.
Blood sample collection
From each subject, 5 mL of blood was drawn from the antecubital vein into tubes containing EDTA. Platelet-free plasma was obtained by centrifugation of blood sample at 2500 rpm for 10-12 minutes and stored at –20ºC until analysis of NO levels. The blood was collected at the time of admission of the patient.
Estimation of plasma total NOx levels
The stable metabolites of NOx, nitrite and nitrate were measured by reduction of nitrate with reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent nitrate reductase combined with detection with the acidic Griess reagent. Fifty microliters of plasma sample/sodium nitrite standard was added to 96-well microtiter plate followed by 40 μl of conversion buffer with nitrate reductase and 10 μl of NADPH solution. Plates were shaken and incubated for 45 minutes at room temperature for nitrate to nitrite conversion. Total nitrite in the sample was determined by Griess assay. Absorbance was measured at 540 nm. The total concentration of nitrite in sample was calculated from the nitrite standard curve. This gives the amount of nitrite stoichiometrically converted from nitrate, plus the originally present nitrite.[17]
Statistical analysis
Data was entered and analyzed using SPSS package, (SPSS 15). Distribution of gender, cigarette smoking, alcohol consumption, hypertension and diabetes between patients and controls were analyzed in the groups by Chi square test/Fishers’ Exact test, and the results are presented as number and percentage. Since plasma total NOx level does not follow normal distribution curve, non-parametric tests, Mann Whitney U/Kruskal Wallis test was used for comparison of plasma total NOx level between groups, and the results are presented as mean ± SD. Spearman's correlation was used to assess the relationship between plasma total NOx level and GOS. The level of significance was fixed at 0.05.
Results
Demographic and clinical characteristics of the patients and controls
A total of 208 patients with aSAH were treated during the study period. Among the patients 113 (54.3%) were females and 95 (45.7%) were males. The age of the patients ranged from 18 to 80 years with an average of 50.47 ± 11.57 years. There were 207 controls with an average of 50.47 ± 12.24 years. Among the risk factors, cigarette smoking and hypertension were found to be significant in the patient group with P < 0.0001 [Table 1]. Logistic regression analysis showed that cigarette smoking and hypertension were independent risk factors for disease outcome.
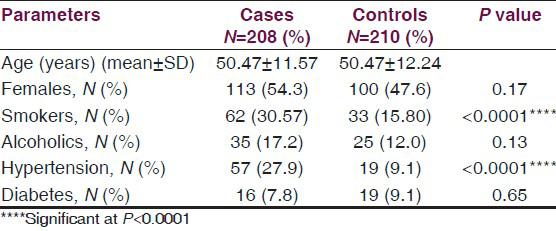
On admission patients were classified according to the Glasgow Coma Scale (GCS). World Federation of Neurosurgeons (WFNS) grading scale was used for clinical assessment of SAH severity [Table 2]. 173 patients underwent surgery (Coiling-151, clipping-19, both coiling and clipping-3) and 35 patients were treated conservatively.
45 (26%) of 173 surgically treated patients were diagnosed with angiographic vasospasm either in the pre operative or post operative period and 33 (73.33%) of 45 patients had clinical vasospasm. [Table 2].
Plasma total NOx levels in different subject groups
There was statistically significant difference in plasma total NOx levels between patients and controls. Plasma level of total NOx in the patients ranged from 2.66 to 53.83 μmol/L, with an average of 19.57 ± 10.09 μmol/L. Plasma total NOx levels of in controls ranged from 6.39 to 202.09 μmol/L, with an average of 41.63 ± 27.78 μmol/L. Therefore, plasma concentration of NOx level was significantly lower in the patients than in the control group (P < 0.001, Figure 1a). The logistic regression analysis indicated plasma NOx level to be an independent predictor aSAH in subjects (OR = 0.908, 95% CI = 0.887 to 0.929, P < 0.0001).
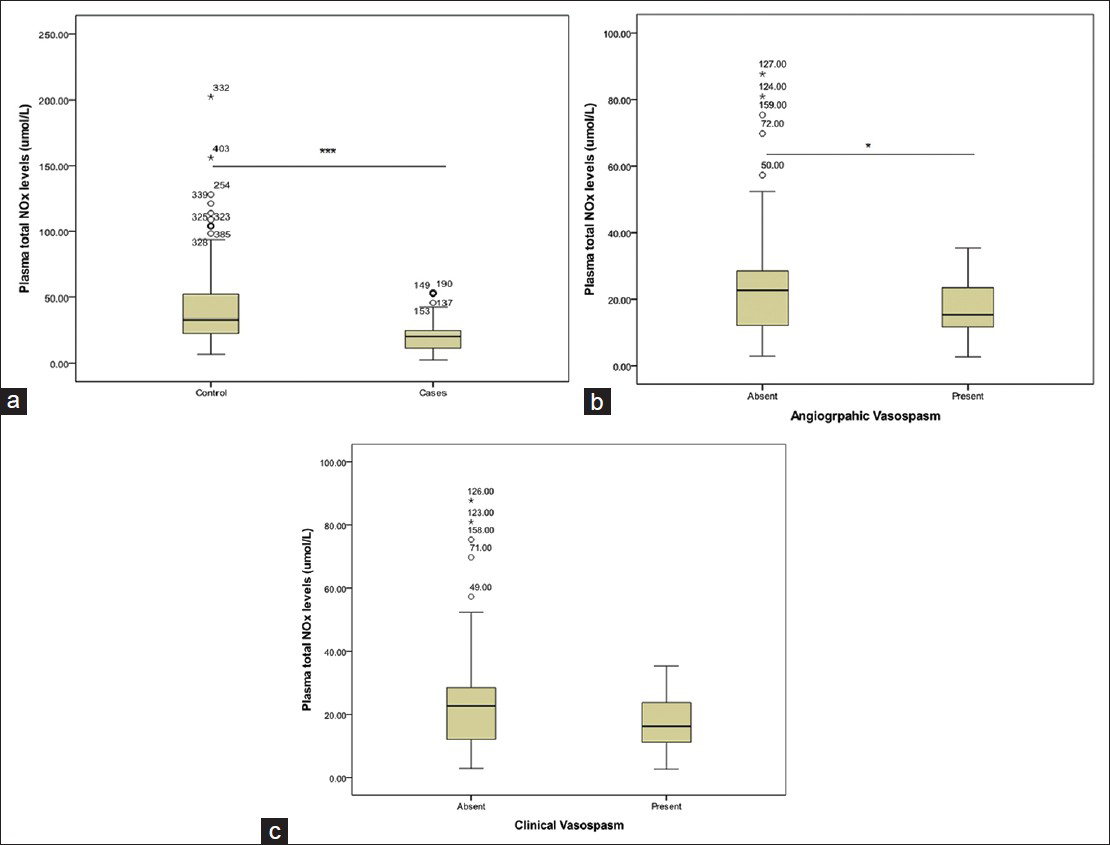
- Graphs showing the level of total nitric oxide (NOx) markers in plasma; (a) in control and SAH patients, (b) in patients with and without angiographic vasospasm, (c) in patients with and without clinical vasospasm. Values are given as mean ± SD (Mann-Whitney test) ***Significant at P < 0.001, *Significant at P < 0.05
Plasma total NOx level in the patients with angiographic vasospasm greatly reduced, ranging from a minimum value of 2.66 to a maximum of 35.32 μmol/L with an average 17.50 ± 7.78 μmol/L. Plasma total NOx levels in patients without vasospasm ranged from 2.84 to 87.65 μmol/L, with an average 23.56 ± 14.80 μmol/L. Hence, there was an association between lower plasma total NOx level and evidence of angiographic vasospasm (P = 0.024, Figure 1b). Patients with clinical vasospasm had a lower NOx levels (18.10 ± 8.14 μmol/L) when compared to patients without vasospasm (23.56 ± 14.80 μmol/L). However, the difference was not significant (P = 0.101, Figure 1c).
Outcome in SAH patients was positively correlated with plasma total NOx levels (correlation coefficient = 0.148, P = 0.035, Figure 2). However, no correlation was observed between WFNS grade and plasma total NOx levels in patients.
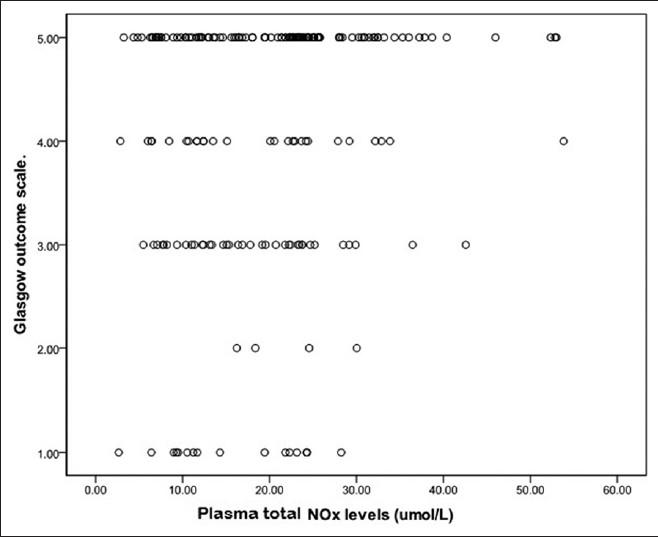
- Graph showing correlation between plasma total NOx concentration and GOS grading system, Values were given as the mean ± SD. Plasma total NOx levels in SAH patients with poor outcome (GOS.1, 2, and 3) was decreased significantly when compared to patients with good outcome (GOS.4 and 5) (Correlation coefficient = 0.148, P = 0.035)
Discussion
Cerebral vasospasm is observed in 70% of the cases of aSAH.[1819] It is as major cause of mortality and morbidity. Predicting vasospasm early may help in early intervention and may improve the outcome after aSAH. Vasospasm develops by third or fourth day following aneurysmal rupture peaking around seventh to ninth day and lasts up to 2-3 weeks. Vasospasm can either be angiographic or symptomatic. Angiographic vasospasm typically refers to arterial narrowing, evident by neuro-imaging findings and 60-70% of the aSAH patients manifest angiographic vasospasm. 30-40% of the patients develop symptomatic or clinical vasospasm. Since symptomatic vasospasm is characterized by the insidious onset of confusion and decreased level of consciousness, followed by focal motor and/or speech impairments it is also known as delayed ischemic neurological deficit (DIND).[213202122]
The oxyhemoglobin, a breakdown product released from the blood clot has more affinity for NOx and scavenges available NOx in the vicinity of the ruptured aneurysm.[1323] Following aSAH there is release of harmful hydroxyl radicals and lipid peroxides which damage the vessel wall endothelium and smooth muscles. Endothelial damage may result in either underproduction of NOx or overproduction of vasoconstrictors like endothelin.[722] Thus, impairing balance between vasoconstrictors and vasodilators resulting in the narrowing of blood vessel leading to cerebral ischemia.[22]
Zubkov et al. have reported endothelial cell apoptosis in the cerebral arteries of a patient who died from vasospasm after SAH. Therefore, endothelial dysfunction or damage due to necrosis or apoptosis may result in reduced generation and release of vasodilators such as NO from these cells. This results in impairment in the equilibrium between vasodilators and constrictors resulting in vasospasm.[24]
Our study confirmed that there is a decrease in the level of plasma total NOx in patients after aSAH compared to healthy controls. Our results are supported by other studies, where they have reported the decrease in nitrate and nitrite concentration in CSF and progressive impairment of NO production rate in the brain after SAH.[2526] We observed that the presence of vasospasm and development of clinical symptoms are strongly associated with lower plasma total NOx levels. Although there was no significant difference between concentration of plasma total NOx and GOS, there was a significant correlation between plasma total concentration of NOx and GOS.
On the contrary, there are studies which show that NO is increased in CSF in SAH-dependent vasospasm.[2728] Woszczyk found a significant elevation of NO metabolites concentration in CSF of patients with increased cerebral blood flow velocity and a delayed neurological deficit as compared to patients without vasospasm. However, the mechanism behind this elevation is attributed to the immunological response after SAH, especially the exposure of the vessel to oxyhemoglobin, causes a reactive increase in expression of the inducible form of NOS (iNOS) in macrophages and activated microglia. The result is a long-lasting overproduction of NO. A high NO concentration may lead to peroxidative injury of cell membranes, resulting in a pathological alteration of the endothelial and the smooth muscle cell layers of the arterial wall. Changes of the cell wall structure may disturb the distribution of NO from endothelial cells to smooth muscle cells. The result is a disruption of the balanced regulation of the cerebral vascular tone, thus causing vasospasm.[28]
Plasma total NOx level is found to be a promising method in predicting development of cerebral vasospasm after aSAH. Efforts are in progress developing therapeutics that can reverse vasoconstriction after aSAH. Studies like intravenous infusion of sodium nitrite in primate models, intraventricular administration of Sodium nitroprusside to aSAH patients with cerebral vasospasm and administration of S-nitroso-N-acetylpenicillamine (SNAP), in experimental vasospasm model in rabbit have successfully reversed cerebral vasospasm.[29303132] Hence, this is promising that the NO donor and precursors could be potential drugs to treat vasospasm in near future.
There are mechanisms that are suspected to be involved in the reduction of NO levels. Polymorphisms in NOSes or increased levels of Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NOSes are thought to deplete the bioavailability of NO in the cerebral arteries.[21333343536] Further research on this realm of science would shed light on the mechanism involved in development of cerebral vasospasm.
Conclusion
In the current study, we analyzed stable metabolites of NO and found a significant decrease in plasma total NOx level in patients with aSAH in Indian population. Furthermore, plasma total NOx level was significantly reduced in patients with cerebral vasospasm. Our results suggest reduced bioavailability of NO as an important mediator in the pathogenesis of cerebral vasospasm. Furthermore, plasma total NOx levels could be used as a candidate biomarker for vasospasm and outcome prediction after aSAH. Determining the exact level of NO that is associated with the triggering of the vasospasm chain would be very useful as a next step in the investigation of this potential marker.
Acknowledgement
We thank ICMR for financial support and we also thank all the volunteers who consented to participate in this study.
Source of Support: Indian Council of Medical Research (ICMR) - Grants.
Conflict of Interest: None declared.
References
- Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid haemorrhage: Putative mechanisms and novel approaches. J Neurosci Res. 2009;87:1-11.
- [Google Scholar]
- Cerebrovascular inflammation following subarachnoid haemorrhage. Jpn J Pharmacol. 2002;88:227-49.
- [Google Scholar]
- Mechanisms of vascular dysfunction after subarachnoid haemorrhage. J Physiol Pharmacol. 2006;57(Suppl 11):145-60.
- [Google Scholar]
- Effect of intraventricular haemorrhage and rebleeding following subarachnoid haemorrhage on CSF eicosanoids. Acta Neurochir (Wien). 1994;129:152-7.
- [Google Scholar]
- Prevention of cerebrovasospasm following subarachnoid haemorrhage in rabbits by the platelet-activating factor antagonist, E5880. J Neurosurg. 1996;84:826-30.
- [Google Scholar]
- Endothelin-1 levels in plasma and cerebrospinal fluid of patients with cerebral vasospasm after aneurysmal subarachnoid haemorrhage. Surg Neurol. 2005;64(Suppl 1):S1-5. discussion S1:5
- [Google Scholar]
- Cerebral vasospasm: A consideration of the various cellular mechanisms involved in the pathophysiology. Neurocrit Care. 2004;1:235-46.
- [Google Scholar]
- Endothelin and subarachnoid haemorrhage: An overview. Neurosurgery. 1998;43:863-76.
- [Google Scholar]
- Acute decrease in cerebral nitric oxide levels after subarachnoid haemorrhage. J Cereb Blood Flow Metab. 2000;20:604-11.
- [Google Scholar]
- Nitric oxide synthase and guanylate cyclase levels in canine basilar artery after subarachnoid haemorrhage. J Neurosurg. 1995;82:250-5.
- [Google Scholar]
- Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-42.
- [Google Scholar]
- Analysis of nitric oxide (NO) in cerebral vasospasm after aneursymal bleeding. Rev Recent Clin Trials. 2007;2:59-67.
- [Google Scholar]
- Effect of intracarotid nitric oxide on primate cerebral vasospasm after subarachnoid haemorrhage. J Neurosurg. 1995;83:118-22.
- [Google Scholar]
- Hydroxylamine attenuates the effects of simulated subarachnoid haemorrhage in the rat brain and improves neurological outcome. Brain Res. 1999;850:225-33.
- [Google Scholar]
- Increased cerebral blood flow but no reversal or prevention of vasospasm in response to L-arginine infusion after subarachnoid haemorrhage. J Neurosurg. 2000;92:121-6.
- [Google Scholar]
- The enzymatic measurement of nitrate and nitrite. In: Titheradge MA, ed. Chapter 9: Methods in Molecular Biology. Totowa, New Jersey: Humana Press Inc; 1997. p. :83-91.
- [Google Scholar]
- Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid haemorrhage: A review. Neurosurgery. 2005;56:633-54.
- [Google Scholar]
- The international cooperative study on the timing of aneurysm surgery. The North American experience. Stroke. 1992;23:205-14.
- [Google Scholar]
- Cerebral vasospasm following aneurysmal subarachnoid haemorrhage. Stroke. 1985;16:562-72.
- [Google Scholar]
- Defining vasospasm after subarachnoid haemorrhage: What is the most clinically relevant definition? Stroke. 2009;40:1963-8.
- [Google Scholar]
- Mechanisms of disease: Roles of nitric oxide and endothelin-1 in delayed cerebral vasospasm produced by aneurysmal subarachnoid haemorrhage. Nat Clin Pract Cardiovasc Med. 2004;1:110-6.
- [Google Scholar]
- A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971-82.
- [Google Scholar]
- Apoptosis of endothelial cells in vessels affected by cerebral vasospasm. Surg Neurol. 2000;53:260-6.
- [Google Scholar]
- Determination of cerebrospinal fluid concentrations of arginine and dimethylarginines in patients with subarachnoid haemorrhage. J Neurosci Methods. 2007;164:155-60.
- [Google Scholar]
- Relation of cerebral energy metabolism and extracellular nitrite and nitrate concentrations in patients after aneurysmal subarachnoid haemorrhage. J Cereb Blood Flow Metab. 2001;21:1067-76.
- [Google Scholar]
- Nitric oxide level and adenosine deaminase activity in cerebrospinal fluid of patients with subarachnoid haemorrhage. Turk Neurosurg. 2008;18:157-64.
- [Google Scholar]
- Nitric oxide metabolites in cisternal CSF correlate with cerebral vasospasm in patients with a subarachnoid haemorrhage. Acta Neurochir (Wien). 2003;145:257-64.
- [Google Scholar]
- Reversal of cerebral vasospasm via intravenous sodium nitrite after subarachnoid haemorrhage in primates. J Neurosurg. 2011;115:1213-20.
- [Google Scholar]
- Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid haemorrhage. JAMA. 2005;293:1477-84.
- [Google Scholar]
- Intraventricular sodium nitroprusside therapy: A future promise for refractory subarachnoid haemorrhage-induced vasospasm. Neurol India. 2003;51:197-202.
- [Google Scholar]
- Reversal of cerebral vasospasm by the nitric oxide donor SNAP in an experimental model of subarachnoid haemorrhage. Acta Neurochir (Wien). 1999;141:1323-9.
- [Google Scholar]
- Endothelial nitric oxide synthase polymorphism (-786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid haemorrhage. Stroke. 2008;39:1103-8.
- [Google Scholar]
- Endothelial nitric oxide synthase gene single-nucleotide polymorphism predicts cerebral vasospasm after aneurysmal subarachnoid haemorrhage. J Cereb Blood Flow Metab. 2008;28:1204-11.
- [Google Scholar]
- The CSF concentration of ADMA, but not of ET-1, is correlated with the occurrence and severity of cerebral vasospasm after subarachnoid haemorrhage. Neurosci Lett. 2012;524:20-4.
- [Google Scholar]
- Association between cerebrospinal fluid levels of asymmetric dimethyl-L-arginine, an endogenous inhibitor of endothelial nitric oxide synthase, and cerebral vasospasm in a primate model of subarachnoid haemorrhage. J Neurosurg. 2004;101:836-42.
- [Google Scholar]






