Translate this page into:
Contemporary Management of Distal Anterior Cerebral Artery Aneurysms: A Dual-Trained Neurosurgeon's Perspective
Sunil V. Furtado, MBBS, MS, MCh, DNB Department of Neurosurgery, MS Ramaiah Medical College and Hospital Bengaluru 560054, Karnataka India sunilvf@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives Distal anterior cerebral artery (DACA) aneurysms are a subset of aneurysms located in the anterior circulation but away from the circle of Willis. We analyze the clinical presentation and outcomes of two treatment groups—surgical and endovascular—for DACA aneurysms managed by a dual-trained neurosurgeon.
Material and Methods A retrospective evaluation of radiological and operative/interventional data of 34 patients with 35 DACA aneurysms over a 12-year period was analyzed. Twenty-seven patients underwent surgery, whereas seven underwent endovascular coiling of the aneurysms. Modified Fisher grade and World Federation of Neurosurgical Societies scale (WFNS) were used to note the subarachnoid hemorrhage (SAH) severity.
Statistical Analysis Categorical data were presented as frequency and percentage, while noncategorical data were represented as mean ± SD. Statistical significance for difference in outcome between the two groups was analyzed using Chi-square test, and p < 0.05 was considered statistically significant.
Results Of 34 patients, 33 presented with a bleed and 23.5% patients were noted to have another aneurysm in addition to the DACA aneurysm. Patients who underwent clipping for another aneurysm along with the DACA aneurysm in a single surgical exercise had a poor outcome compared with those who underwent surgery for the lone DACA aneurysm (7 vs. 20, p = 0.015). Most patients in both surgical (70.37%) and endovascular (85.71%) groups had good outcome (mRS ≤ 2).
Conclusions A good outcome can be achieved with either surgery or endovascular coiling in the management of DACA aneurysms. In patients with multiple aneurysms, SAH with aneurysmal rupture of DACA should be managed first; the other unruptured aneurysm may be operated after an interval to avoid morbidity.
Keywords
aneurysm
clipping
distal anterior cerebral artery
endovascular coiling
subarachnoid hemorrhage
Introduction
Distal anterior cerebral artery (DACA) aneurysms are defined as aneurysms located on the A2-A5 segments of the anterior cerebral artery and on its distal branches.1 2 3 4 DACA aneurysms account for 2 to 9.2% of all intracranial aneurysms.1 2 They are relatively uncommon aneurysms, located distal to the circle of Willis, characterized by a small size, and associated with a broad base with originating branches and anatomical anomalies of the anterior cerebral artery.2 Their clinical presentations are different from other proximally located aneurysms on the intracranial anterior circulation. Although a meta-analysis on the outcome of surgical and endovascular management of these aneurysms has been published, there are few publications on the management of these aneurysms by a dual-trained neurosurgeon.5 We discuss the clinical presentation; evaluate the outcome of surgical and endovascular treatment of these aneurysms in relation to demographics, clinical status at presentation, and grade of subarachnoid hematoma (SAH)/bleed on CT scan or MR imaging. The various endovascular modalities of aneurysm treatment are also discussed here.
Materials and Methods
The study population was identified through a retrospective review of radiological imaging, which included digital subtraction angiogram (DSA) images, operative videos and surgical notes related to all DACA aneurysms. Patients with DACA aneurysm, aged between 19 to 72 years were included in this study, numbering a total of 34 cases, categorized into two groups, namely, surgical and endovascular coiling group, based on the treatment modality adopted. The surgical group comprised 27 patients constituting 3.2% of the aneurysms operated over a 12-year period between January 2007 and June 2019. Endovascular coiling group consisted of seven patients and were managed using the endovascular route over a period of 4 years and 6 months from January 2015 to June 2019. All patients in both the treatment arms were managed by a single dual-trained neurosurgeon. Demographic data such as age and sex and findings of MRI and CT angiogram were collected from medical electronic records and radiological reports, respectively.
All patients had undergone DSA prior to intervention. Patients in the surgical group were operated through either a pericoronal parasagittal craniotomy and an interhemispheric corridor approach, extended anterior interhemispheric approach, or extended frontotemporal craniotomy. Neuronavigational guidance (Medtronic Stealth neuronavigation system) was used in the surgical group. For patients who were managed by endovascular coiling, either Barricade or Axiem coils were used for the procedure. The aneurysms were coiled using a 6-Fr guide catheter and a 0.0165” (Vasco + 10 microcatheter, Balt USA, Irvine, CA) or 0.017” (Echelon 10 microcatheter, Medtronic Neurovascular, Irvine, CA) microcatheter placed in them guided by a 0.014” microwire. The severity of SAH was assessed, based on the modified Fisher grade and World Federation of Neurosurgical Societies scale (WFNS).6 7 Outcome at follow-up was evaluated using the modified Rankin scale (mRS).8 A good outcome was defined as a mRS of ≤ 2. Patients who underwent endovascular coiling of the DACA aneurysm were scored, based on the Raymond Roy occlusion classification for aneurysm occlusion postcoiling, as presented in Fig. 1.9
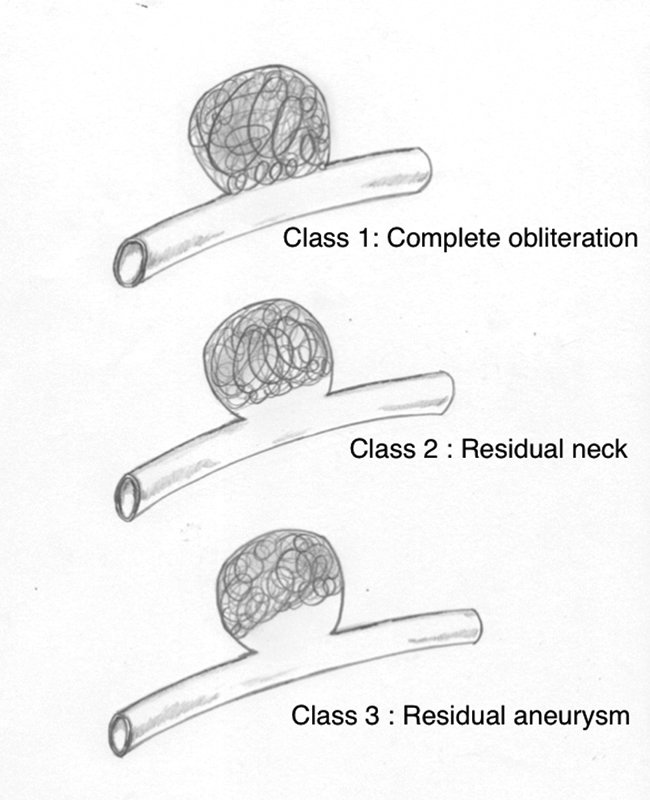
-
Fig. 1 Raymond Roy occlusion classification.
Fig. 1 Raymond Roy occlusion classification.
Per our protocol, patients underwent a DSA or CT angiogram at 3-month follow-up after the intervention. A DSA was the preferred modality of evaluation of the postprocedure status of the aneurysm. A CT angiogram was performed in case the patient turned down a DSA test. CT angiogram was performed at 1- and 2-year follow-up after surgery.
Statistical Analysis
Statistical analyses were performed with SPSS for Windows (SPSS; SPSS Inc., Chicago, IL). Categorical data were presented as frequency and percentage, while noncategorical data were represented as mean ± SD. Statistical significance for difference in outcome between the two groups was analyzed using Chi-square test, and p < 0.05 was considered statistically significant.
Results
The mean age of the patients was 48.9 ± 13.03 years (range 19–72 years, median 47 years). In the surgical group, the majority were male (51.85%), presented with DACA aneurysm bleed (92.59%), and were less than 50 years of age (59.25%). The majority in the endovascular treatment group were females (85.71%), aged > 50 years (71.43%), and all presented with DACA aneurysm bleed (100%), as presented in Table 1. Seven patients in the surgical group had other aneurysms along with a DACA aneurysm—anterior communicating aneurysm (3), middle cerebral aneurysm (2), ophthalmic segment aneurysm (1), and bilateral DACA aneurysms (1). This adds up to 20.5% of patients with multiple aneurysms associated with DACA aneurysm (Table 1).
|
Variables |
Number of patients n (%) |
||
|---|---|---|---|
|
Surgical group (n = 27) |
Endovascular group (n = 7) |
||
|
Gender |
Male |
14 (51.85) |
1 (14.29) |
|
Female |
13 (48.15) |
6 (85.71) |
|
|
Age (years) |
< 50 |
16 (59.25) |
2 (28.57) |
|
> 50 |
11 (40.75) |
5 (71.43) |
|
|
Indication |
DACA bleed |
25 (92.59) |
7 (100) |
|
Vertigo |
1 (3.7) |
0 |
|
|
Other SAH |
1 (3.7) |
0 |
|
|
Additional aneurysm |
DACA only |
20 (74) |
7 (100) |
|
DACA + anterior communicating aneurysm |
3 (11.11) |
0 |
|
|
DACA + middle cerebral aneurysms |
2 (7.4) |
0 |
|
|
DACA + ophthalmic segment aneurysm |
1 (3.74) |
0 |
|
|
Bilateral DACA aneurysms |
1 (3.74) |
0 |
|
|
Mode of surgery |
Parasagittal craniotomy and an interhemispheric corridor |
21 (77.78) |
– |
|
Extended frontotemporal craniotomy |
5 (18.52) |
– |
|
|
Anterior interhemispheric approach |
1 (3.7) |
– |
|
|
Raymond Roy occlusion classification |
1 |
– |
5 (71.43) |
|
2 |
– |
2 (28.57) |
|
|
WFNS |
1 |
22 (81.48) |
3 (42.86) |
|
2 |
0 |
2 (28.57) |
|
|
3 |
3 (11.11) |
1 (14.29) |
|
|
4 |
2 (7.41) |
1 (14.29) |
|
|
Modified Fisher grade |
1 |
2 (7.41) |
0 |
|
2 |
11 (40.74) |
5 (71.43) |
|
|
3 |
10 (37.04) |
2 (28.57) |
|
|
4 |
4 (14.81) |
0 |
|
|
Aneurysm site |
Right |
17 (62.96) |
4 (57.14) |
|
Left |
9 (33.33) |
3 (42.86) |
|
|
Bilateral |
1 (3.7) |
0 |
|
|
Size of aneurysm |
Small (< 1cm) |
20 (74.07) |
6 (85.71) |
|
Large (1–2.4 cm) |
6 (22.22) |
1 (14.29) |
|
|
Giant |
1 (3.7) |
0 |
|
|
New postoperative deficits |
Absent |
20 (74.07) |
– |
|
Weakness |
7 (25.93) |
– |
|
|
mRS outcome |
Good (0–2) |
19 (70.37) |
6 (85.71) |
|
Poor (3–6) |
8 (29.63) |
1 (14.29) |
|
Abbreviations: DACA, distal anterior cerebral artery; mRS, modified Rankin scale; SAH, subarachnoid hemorrhage; WFNS, World Federation of Neurosurgical Societies Scale.
Majority of patients in both groups had WFNS grade 1 (surgical: 81.48% and endovascular: 42.86%) and modified Fisher grade 2 bleed (surgical: 40.74% and endovascular: 71.43%) on CT/MR imaging at presentation and had right side aneurysm (surgical: 62.96% and endovascular: 57.14%) and small size aneurysm of less than 1 cm (surgical: 74.07% and endovascular: 85.71%), as presented in Table 1. Out of the small aneurysms, 13 (48%) in the surgical group and 3 (42.8%) in the endovascular group were less than 6 mm size, classified as very small aneurysm.10 Among the patients who had Fisher grade 3 and 4 bleed, 9 (33%) had intracerebral hematomas (ICH). Anatomical variants were seen in three patients in the surgical group, of whom two patients had an azygous anterior cerebral artery. One patient had infraoptic origin of the anterior cerebral artery associated with an anterior communicating artery aneurysm and a DACA aneurysm at the junction of the right pericallosal and callosomarginal artery.
The mean age of patients in the surgical group was 48.1 years with a median of 47 years. Most patients in the surgical group were operated through parasagittal craniotomy and an interhemispheric corridor (77.78%) to reach the aneurysm. No new neurological deficits were noted in 74% of operated patients. Among the seven surgical patients who developed weakness in the postoperative period, two were attributed to vasospasm and one patient had a right frontal venous infarct and superior mesenteric artery (SMA) syndrome. Five patients underwent an extended frontotemporal craniotomy extending to the midline, in order to secure aneurysms at both locations. One patient with SAH, and aneurysms in the anterior communicating artery and DACA underwent an “extended” anterior interhemispheric approach to clip both the aneurysms. One patient who presented with a ruptured ophthalmic segment aneurysm was operated for the same at presentation, and also had an unruptured DACA aneurysm which was subsequently operated at 6-month follow-up. Five (18.5%) patients had a postoperative retraction associated frontal contusion, which did not require any further intervention. Out of these, four patients had another aneurysm operated in addition to the DACA aneurysm. Fig. 2 is an illustrative case of a large DACA aneurysm.
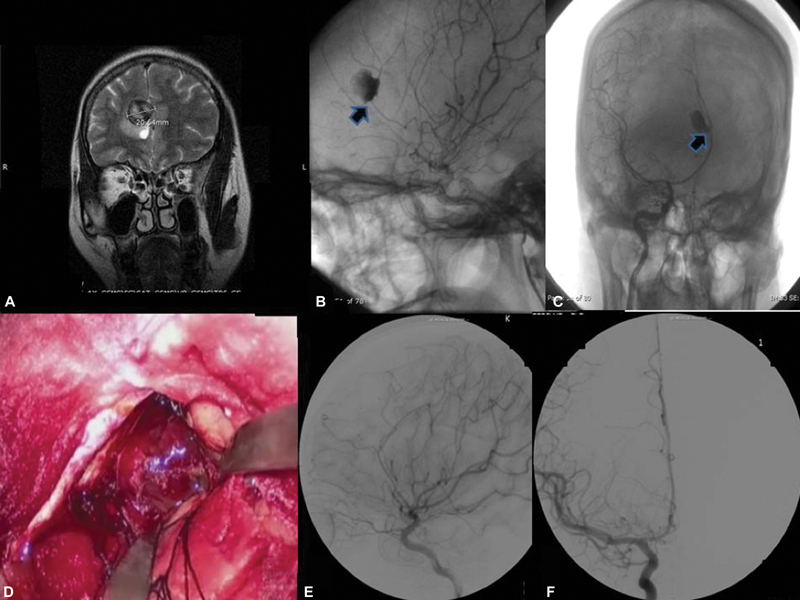
-
Fig. 2 (A) Coronal MRI brain showing a large distal anterior cerebral artery aneurysm with luminal thrombus; (B) and (C) Digital subtraction angiogram (DSA) images lateral and anteroposterior (AP) view of the aneurysm; (D) Intraoperative picture of the aneurysm; (E) and (F) DSA images lateral and AP view showing aneurysm exclusion postclipping.
Fig. 2 (A) Coronal MRI brain showing a large distal anterior cerebral artery aneurysm with luminal thrombus; (B) and (C) Digital subtraction angiogram (DSA) images lateral and anteroposterior (AP) view of the aneurysm; (D) Intraoperative picture of the aneurysm; (E) and (F) DSA images lateral and AP view showing aneurysm exclusion postclipping.
In the surgical group, the mean duration of stay was 19.7 days (median 15 days). Twenty-three patients were on follow-up for a mean duration of 10.8 months (range 3 months to 41/2 years). Fourteen (60%) of the 23 patients had a check DSA, and 5 (21.7%) underwent a CT angiogram at 3-month follow-up. There was no residual aneurysm located at the DACA operative site. Twelve of these patients underwent CT angiogram at 1 year and five patients at 2-year follow-up after surgery, with no recurrence noted on imaging.
There were no mortalities recorded in the peroperative period. Nineteen (82%) of the 23 patients had a good outcome at last follow-up. Age, sex, hemispheric side of the aneurysm, time duration from presentation to surgery, number of clips used at surgery, WFNS grade at presentation, and modified Fisher score at presentation CT/MR imaging had no bearing on patient outcome. Patients with multiple aneurysms (DACA aneurysm with an aneurysm at a different location, including bilateral DACA aneurysms), presenting with SAH and having both the DACA and the other aneurysm clipped in the same sitting, had a statistically poor outcome than those who had undergone surgery for a lone DACA aneurysm (7 vs. 20, p = 0.015). This is illustrated in Fig. 3. There was no significant statistical difference in the outcome between the surgical group when analyzed over patient demographics, duration from presentation to intervention, clinical/radiological presentation, and aneurysm location (p > 0.05). In the endovascular treatment group, coiling was attempted for a patient with a DACA aneurysm. It was abandoned in favor of aneurysm clipping, as it was not possible to maintain the microcatheter inside the aneurysm during coiling, due to a relatively wide neck. The decision for endovascular intervention was based on the review of the preoperative CT angiogram or DSA images and the possible support the extracranial and intracranial vasculature would provide to the endovascular hardware systems for aneurysm coiling.
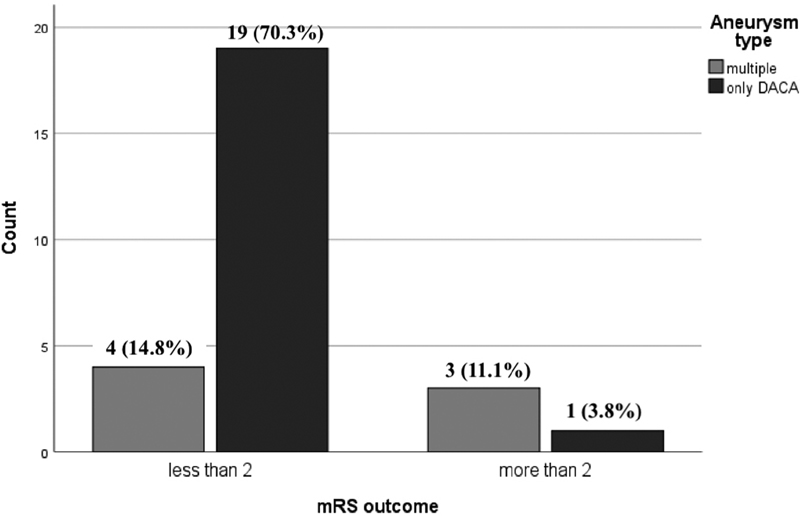
-
Fig. 3 Comparison of surgical outcome of solitary distal anterior cerebral artery (DACA) aneurysm-only group with DACA with another aneurysm group.
Fig. 3 Comparison of surgical outcome of solitary distal anterior cerebral artery (DACA) aneurysm-only group with DACA with another aneurysm group.
The mean age of endovascular treatment group was 51.5 years with a median age of 54 years. No intraprocedural ruptures were noted among these patients. The mean duration of hospital stay was 15.6 days (median 11 days) and the mean follow-up period was 9 months (range: 5 months to 2 years and 4 months; median: 10 months). Majority of patients had Raymond Roy class 1 (71.43%) postcoiling, as presented in Fig. 4. Six (85%) of the seven patients were in mRS grade 0 at follow-up, indicating good outcome. However, one (15%) patient had poor outcome. He had a large hematoma, WFNS grade 4, modified Fisher grade 4 at presentation, and a mRS of 4 at 1-year follow-up. Five patients had undergone a CT angiogram and one patient a DSA at follow-up period, ranging from 3 months to 2 years after aneurysm coiling. Two patients had coil compaction with a visible aneurysm neck in follow-up imaging. They declined further management. Since this was a small subgroup of patients, we did not analyze outcome based on patient demographics and clinical and radiological presentations.
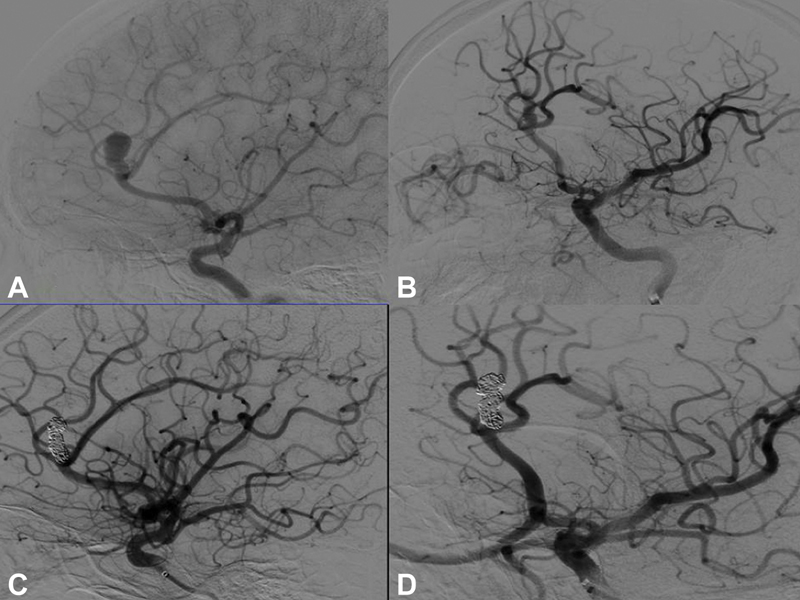
-
Fig. 4 (A, B) Digital subtraction angiogram (DSA) lateral and oblique views of left distal anterior cerebral artery aneurysm at the origin of pericallosal and callosomarginal artery; (C) and (D) Postprocedure DSA lateral and oblique views Raymond Roy class 1 occlusion.
Fig. 4 (A, B) Digital subtraction angiogram (DSA) lateral and oblique views of left distal anterior cerebral artery aneurysm at the origin of pericallosal and callosomarginal artery; (C) and (D) Postprocedure DSA lateral and oblique views Raymond Roy class 1 occlusion.
Discussion
DACA aneurysms were reported to have a poor management outcome in comparison to aneurysms at other locations and were often referred to as malignant aneurysms.4 11 A study conducted over a 25-year period reported an incidence of 6.2% for DACA aneurysms out of 2137 aneurysms operated, and A3 segment of the anterior cerebral artery was the most common location of the DACA aneurysm, representing 56% of the cases.2 This study noted a lower incidence of 3.8% for DACA aneurysms in our aneurysm case series. Ninety-seven percent of the patients presented with a bleed attributed to the DACA aneurysm, and there were no traumatic or mycotic aneurysms in the series. Twenty-six (76.4%) of the aneurysms in our series were small sized (< 1 cm).
The incidence of ICH related with ruptured DACA aneurysm is reported to vary between 40% to 70% in previous literature.11 12 13 We noted an incidence of 33% of ICH, which was lower than reports of Ohno et al and Sekerci et al who reported an incidence of 40% and 70% of ICH, respectively.11 12 This may be due to the aneurysm located between a firm falx and frontal lobe, with little intervening subarachnoid space. Orz reported an incidence of 86% for ruptured small DACA aneurysms, indicating that these aneurysms may be intervened on despite their small size.9 13 Researchers also reported poor admission grade and initial ICH as factors for unfavorable prognosis.
Surgery was performed mostly through the parasagittal route, followed by retraction of the frontal lobe away from the falx. A single retractor was used in all cases with no retractor placed on the falx. Dissection was performed in a direction proximal to the supposed site of the aneurysm to secure proximal control. Neuronavigational guidance was used in 11 (40.7%) cases. Aneurysm rupture was encountered in 4 (14.8%) cases during dissection and was controlled by application of a proximal clip, and then further continuation of aneurysm neck dissection and clipping. A higher intraoperative rupture of 35% was reported by Orz.13 In this study, patients who underwent surgery for another aneurysm in addition to the DACA aneurysm fared statistically more poorly than patients who underwent surgery for the ruptured singular DACA aneurysm (p < 0.05). The possible contributors to poor outcome might be a larger craniotomy, extensive subarachnoid dissection at two locations, concomitant vessel manipulation due to aneurysm location on the anterior communicating artery and bilateral DACA (n = 4), and quantum of bleed.
Endovascular treatment: Coiling as a modality of treatment was previously advocated for patients with poor-grade SAH.11 14 However, with advancements in DSA radiology and endovascular hardware technologies, it is easier to visualize and catheterize beyond the aneurysm neck.15 Although we had seven patients in the endovascular treatment group, 85% of the patients had a good outcome at last follow-up. As much as 71.6% had Raymond Roy grade 1 or 2 aneurysm occlusion at completion of the coiling procedure. Similar reports of Raymond Roy grade 1 or 2 aneurysm occlusion were reported in previous studies.16 17 Suzuki et al and Sturiale et al reported a rate of 66.3% and 78%, respectively, at completion of coiling procedure for DACA aneurysms.16 17 Both noted a coil compaction at follow-up angiogram. Conversely, Huang et al had an impressive success rate of 97% at the end of the coiling procedure.1 However, the clearance of the intracerebral bleed and thick interhemispheric SAH is not possible by the endovascular route. We have moved toward management of DACA aneurysms through the endovascular route only when the proximal vasculature can support aneurysm catheterization.
Pipeline Embolization Device, Silk Vista Baby stent, and Flow-Redirection Endoluminal Device (FRED) have been used as stent-flow diverters for these aneurysms under coverage of dual antiplatelet agents.18 19 These studies had employed the use of Flow diverter stent placement in treating unruptured DACA aneurysms. Cagnazzo et al reported that 3 out of 15 treated patients developed ischemic complications in the postprocedure period. Aneurysm exclusion was seen in 70% of their study.18 We do not have experience of treating DACA aneurysms with flow diversion.
In the surgical arm of our study, 25.9% of the patients developed postoperative weakness due to vasospasm. Retraction contusion and venous infarct was noted in 22.2% of operative cases in our study, underlying the tight corridor for dissection around important venous drainage channels and retraction on an edematous brain. Reexploratory surgery for decompression craniotomy was not needed to be performed for these complications. Furthermore, Lehenka et al mentioned a surgical morbidity and mortality rate of 1% and 12%, respectively.3 Their 1-year mortality rate was 13% and a favorable outcome was observed in 78%. There was no mortality noted in the present study.
In terms of outcome, 82% of our surgical group patients and 85% of the endovascular group patients had a good treatment outcome at last follow-up. A study conducted by Peschillo et al reported a good outcome with an mRS score of 0 to 2 in surgical (67.4%) and endovascular (78.9%) groups (p = 0.034).20 Hunt and Hess grade, rebleed before treatment, ICH and intraventricular hemorrhage, and severe preoperative hydrocephalus have been reported as independent factors that can predict the unfavorable outcome at 1-year follow up.3 Pandey et al showed that patients who had undergone clipping of DACA aneurysms had a better outcome than the endovascular group. As much as 92% of the surgical group versus 64% in the endovascular group had a good outcome at follow-up, although the results were not statistically significant (p = 0.36). The study had 28 patients undergoing endovascular coiling and 13 microsurgical clipping.21 In contrast, our study had a larger surgical arm, of which 70.3% demonstrated a good outcome. We included management of other unruptured aneurysms along with the DACA aneurysm and emphasized the uniqueness of a single operator on both the study arms.
There are some limitations to this study. Most patients in the surgical group were of good clinical grade (WFNS grade 1) at presentation. This could be due to a referral bias, as many patients were referred from other parts of the country and a good WFNS grade could facilitate such travel. The endovascular group was smaller than the operative group (7:27). We have not characterized the location of the aneurysm on the DACA segment. Patients with a poor mRS at discharge and who were lost to follow-up may have succumbed either to SAH-related management complications or to secondary causes at an outside rehabilitation facility within or after the 30-day period after aneurysm treatment. As this was a retrospective study, such details were unavailable.
Conclusion
In the surgical management of the ruptured DACA aneurysms, the DACA aneurysm should be operated first, followed by a later procedure on the other unruptured aneurysm. The management of DACA aneurysms need to be individualized, based on aneurysm morphology and vascular anatomy proximal to the aneurysm, which can aid in aneurysm access. In case of unfavorable proximal vasculature, the surgical option can be chosen.
Conflict of Interest
None declared.
Funding None.
References
- Endovascular treatment of ruptured distal anterior cerebral artery aneurysm. Neurol India. 2010;58(2):259-263.
- [Google Scholar]
- Microsurgical treatment of distal anterior cerebral artery aneurysms: A 25 year institutional experience. Neurol India. 2016;64(6):1204-1209.
- [Google Scholar]
- Anatomic features of distal anterior cerebral artery aneurysms: a detailed angiographic analysis of 101 patients. Neurosurgery. 2008;63(2):219-228. , discussion 228–229
- [Google Scholar]
- Outcome in 43 patients with distal anterior cerebral artery aneurysms. Stroke. 1997;28(12):2405-2409.
- [Google Scholar]
- Safety and efficacy of surgical and endovascular treatment for distal anterior cerebral artery aneurysms: a systematic review and meta-analysis. World Neurosurg. 2017;100:557-566.
- [Google Scholar]
- Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21-27. , discussion 21–27
- [Google Scholar]
- A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51(11):1457.
- [Google Scholar]
- Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. 2017;48(7):2007-2012.
- [Google Scholar]
- An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 2015;7(7):496-502.
- [Google Scholar]
- Rupture of very small intracranial aneurysms: incidence and clinical characteristics. J Cerebrovasc Endovasc Neurosurg. 2015;17(3):217-222.
- [Google Scholar]
- Aneurysms of the distal anterior cerebral artery: a clinical series. Neurol Neurochir Pol. 2011;45(2):115-120.
- [Google Scholar]
- Saccular aneurysms of the distal anterior cerebral artery. Neurosurgery. 1990;27(6):907-912. , discussion 912–913
- [Google Scholar]
- Surgical Strategies and outcomes for distal anterior cerebral arteries aneurysms. Asian J Neurosurg. 2011;6(1):13-17.
- [Google Scholar]
- Clinical features and surgical outcomes of ruptured distal anterior cerebral artery aneurysms in 20 consecutively managed patients. J Clin Neurosci. 2009;16(6):802-806.
- [Google Scholar]
- Use of the microangiographic fluoroscope for coiling of intracranial aneurysms. Neurosurgery. 2011;69(5):1131-1138.
- [Google Scholar]
- Endovascular treatment of distal anterior cerebral artery aneurysms: single-center experience and a systematic review. AJNR Am J Neuroradiol. 2013;34(12):2317-2320.
- [Google Scholar]
- Endovascular therapy of distal anterior cerebral artery aneurysms: single- institution clinical experience with 47 patients (49 aneurysms) Journal of Neuroendovascular Therapy. 2019;13:329-335.
- [Google Scholar]
- Treatment of distal anterior cerebral artery aneurysms with flow-diverter stents: a single-center experience. AJNR Am J Neuroradiol. 2018;39(6):1100-1106.
- [Google Scholar]
- Flow diversion beyond the circle of Willis: endovascular aneurysm treatment in peripheral cerebral arteries employing a novel low-profile flow diverting stent. J Neurointerv Surg. 2019;11(12):1227-1234.
- [Google Scholar]
- A systematic review and meta-analysis of treatment and outcome of blister-like aneurysms. AJNR Am J Neuroradiol. 2016;37(5):856-861.
- [Google Scholar]
- Management of distal anterior cerebral artery aneurysms: a single institution retrospective analysis (1997-2005) Neurosurgery. 2007;61(5):909-916. , discussion 916–917
- [Google Scholar]






