Translate this page into:
Clinical and neuroimaging features of isolated neurological melioidosis: A series of five patients
*Corresponding author: Dr. Molly M. Thabah, Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. mollymthabah@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saibaba J, Sunitha VC, Bammigatti C, Nagarajan K, Mary Thabah M. Clinical and neuroimaging features of isolated neurological melioidosis: A series of five patients. J Neurosci Rural Pract. doi: 10.25259/JNRP_397_2024
Abstract
Neurological melioidosis, caused by Burkholderia pseudomallei, is a rare but serious manifestation of melioidosis that can lead to significant morbidity and mortality. This case series highlights the clinical presentations, neuroimaging findings, and management outcomes of five adult patients diagnosed with isolated neurological melioidosis between January 2019 and June 2023. All patients exhibited subacute meningoencephalitis, hemiparesis, and cerebellar signs. Neuroimaging studies revealed distinctive T2 hyperintensities and ring-enhancing lesions affecting the corticospinal tract, internal capsule, and cerebellum. The diagnosis was confirmed through positive serological tests for B. pseudomallei. Treatment with meropenem and supportive care resulted in clinical improvement in four patients, while one patient died due to the disease. This series underscores the importance of considering isolated neurological melioidosis in patients presenting with subacute meningoencephalitis, especially in endemic areas. Early diagnosis, supported by neuroimaging and serological testing, along with timely antibiotic therapy, is crucial for improving outcomes and reducing mortality in these cases.
Keywords
Burkholderia pseudomallei
Neuroimaging
Neuromelioidosis
Tract sign
Tunnel sign
INTRODUCTION
Melioidosis, caused by the aerobic, intracellular, Gram-negative bacterium Burkholderia pseudomallei, is a potentially life-threatening infectious disease. This soil-dwelling pathogen is endemic in tropical and subtropical regions, particularly in Southeast Asia and northern Australia, where environmental conditions such as high humidity and rainfall favor its survival. B. pseudomallei can infect humans through direct inoculation, inhalation, or ingestion, often leading to a wide spectrum of clinical manifestations. These range from acute sepsis and pneumonia to chronic localized infections involving the skin, soft tissues, and visceral organs. In severe cases, the disease can progress to deep-seated abscess formation and multiorgan dysfunction, making early diagnosis and treatment critical to improving outcomes. Among the various clinical presentations of melioidosis, neurological involvement is relatively rare and can manifest as encephalomyelitis, brain abscesses, or meningoencephalitis, with or without cerebellar signs. Due to its nonspecific clinical features, neurological melioidosis can be challenging to diagnose, particularly in non-endemic regions where clinicians may have limited familiarity with the disease and carry high morbidity and mortality. In such cases, neuroimaging plays a pivotal role in identifying characteristic findings, such as T2 hyperintensities and ring-enhancing lesions, which can guide further diagnostic workup and prompt initiation of appropriate therapy.
Here, we present five patients with isolated neurological diseases where imaging had an important role in diagnosis and initiation of management.
CASE SERIES
Five patients (four males and one female) with a mean age of 23 years (range 15–38 years) were admitted between January 2019 and June 2023 with a confirmed diagnosis of melioidosis. The clinical features are briefly presented below.
Patient 1
A 15-year-old boy presented with a fever accompanied by a headache over two months and right hemiparesis for two weeks. On examination, right-side hemiplegia and neck stiffness were present. On initial suspicion of chronic meningitis, cerebrospinal fluid (CSF) analysis showed predominantly mononuclear pleocytosis [Table 1]. Magnetic resonance imaging (MRI) of the brain revealed hyperintensities (T2/Fluid-Attenuated Inversion Recovery [FLAIR] sequences) in (a) the cerebral peduncle, (b) the posterior limb of the internal capsule, and (c) bilateral perirolandic region [Figure 1]. The hyperintensities are tracking along the corticospinal tract up to the level of cerebral peduncles. This finding is characteristic of neurological melioidosis. Indirect hemagglutination assay (IHA) on CSF was positive for B. pseudomallei. Treatment with intravenous (IV) meropenem and ceftazidime [Table 1] led to clinical improvement. Follow-up MRI after six weeks demonstrated a reduction in signal changes.
| Patient number | Age/Sex | Syndromic presentation | MRI findings (brain + spine) | CSF analysis | ||
|---|---|---|---|---|---|---|
| Cell count (cells/µL) (Mononuclear cells%) | CSF glucose (mg/dL) | Protein (mg/dL) | ||||
| 1 | 15 year/Male | Acute meningoencephalitis and right hemiparesis | Axial section T2/FLAIR hyperintensities in (a) cerebral peduncle, (b) posterior limb of internal capsule and (c) bilateral perirolandic region [Figure 1] | 180 (61) | 66 | 52 |
| 2 | 28 year/Male | Acute meningoencephalitis, cerebellar signs and lower Cranial nerve palsies | Coronal section T2/FLAIR hyperintensities in (d) left corticospinal tract, (e) left pons and medulla and (f) right cortico-ponto-cerebellar tract [Figure 1] |
80 (100) | 67 | 101 |
| 3 | 23 year/Male | Subacute meningoencephalitis, right hemiparesis, cerebellar signs | Axial section T2/FLAIR hyperintensities in (g) left cerebellum, left middle cerebellar peduncle, (h) Coronal section involving brainstem, (i) bilateral corticospinal tract, and high frontoparietal region [Figure 1] | 80 (60) | 77 | 28 |
| 4 | 15 year/Female | Subacute meningoencephalitis, asymmetric cerebellar signs, transverse myelitis | Neuroimaging can be divided into three stages – early stage, acute stage (after 10 days), and recovery stage. In early stage, (a) axial section T2/FLAIR hyperintensities in the right cerebellum and (b) coronal section T2/FLAIR hyperintensities in the right middle cerebellar peduncle. Acute stage (After 10 days of onset): (c) Axial section T2/FLAIR hyperintensities in the right pons, (d) bilateral posterior limb of internal capsule, (e) bilateral corona radiata, (f) bilateral centrum semiovale, (g) bilateral cerebellar peduncle, (h) cervical spine to cauda equina in spinal cord. MRI brain coronal section showing (i) tract sign-T2/FLAIR hyperintensities along bilateral diffuse corticospinal tract. Recovery phase (At three months follow-up post-treatment with Meropenem+TMP-SMX)-Resolving T2/FLAIR hyperintensities along (j) bilateral corticospinal tract – axial section and (k) coronal section and (l) cervical spinal cord [Figure 2] |
130 (90) | 75 | 86 |
| 5 | 38 year/Male | Acute meningoencephalitis, right hemiparesis, cerebellar signs | Axial T2/FLAIR hyperintensity in medulla, pons (confluent), midbrain, upper cervical cord, cerebellar peduncles, bilateral internal capsule, right corona radiata, centrum semi-ovale, patchy contrast enhancement | 250 (16) | 72 | 92 |
| 1 | 15 year/Male | Sterile | Positive | Ceftazidime 6 g/day (initial) followed by meropenem 6 g/day for total six weeks. Maintenance with TMP-SMX:800/160 mg for six months |
Full recovery | |
| 2 | 28 year/Male | Sterile | Positive | Meropenem 6 g/day× 6 weeks Maintenance with TMP-SMX: 800/160 mg for six months |
Full recovery | |
| 3 | 23 year/Male | Sterile | Negative | Ceftazidime 6 g/day Maintenance with TMP-SMX: 800/160 mg for six months | Neurologic impairment | |
| 4 | 15 year/Female | Sterile | Positive | Meropenem 6 g/day× 6 weeks Maintenance with TMP-SMX: 800/160 mg for six months |
Full recovery | |
| 5 | 38 year/Male | Sterile | Negative | Meropenem 6 g/day× 6 weeks Maintenance with TMP-SMX: 800/160 mg for six months |
Neurologic impairment | |
Normal reference range: CSF cell counts: <5 cells/µL, CSF protein 15–45 mg/dL, CSF Glucose 45–80 mg/dL. CSF: Cerebrospinal fluid, MRI: Magnetic resonance imaging, TMP-SMX: Trimethoprim-sulfamethoxazole (Co-trimoxazole), FLAIR: Fluid-attenuated inversion recovery.
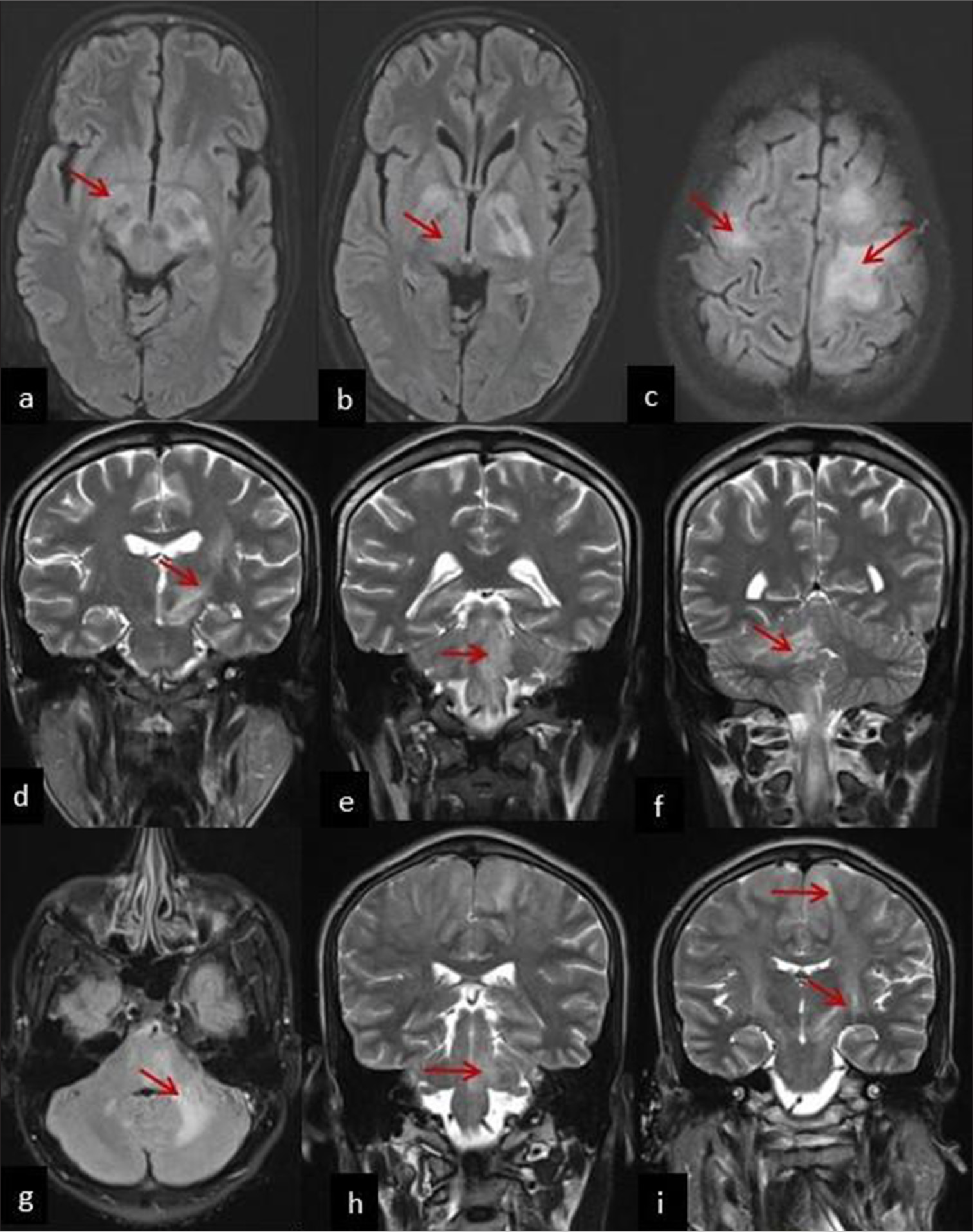
- Magnetic resonance imaging (MRI) brain axial section T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in (a) cerebral peduncle (red arrow), (b) posterior limb of internal capsule (red arrow), and (c) bilateral perirolandic region (red arrow) (Patient 1), MRI brain coronal section shows T2/FLAIR hyperintensities in (d) the left corticospinal tract (red arrow), (e) left pons and medulla (red arrow), and (f) right cortico-ponto-cerebellar tract (red arrow) (Patient 2), MRI brain axial section T2/FLAIR hyperintensities in (g) the left cerebellum, left middle cerebellar peduncle (red arrow), (h) coronal section involving the brainstem (red arrow), and (i) bilateral corticospinal tract and high frontoparietal region (red arrow) (Patient 3).
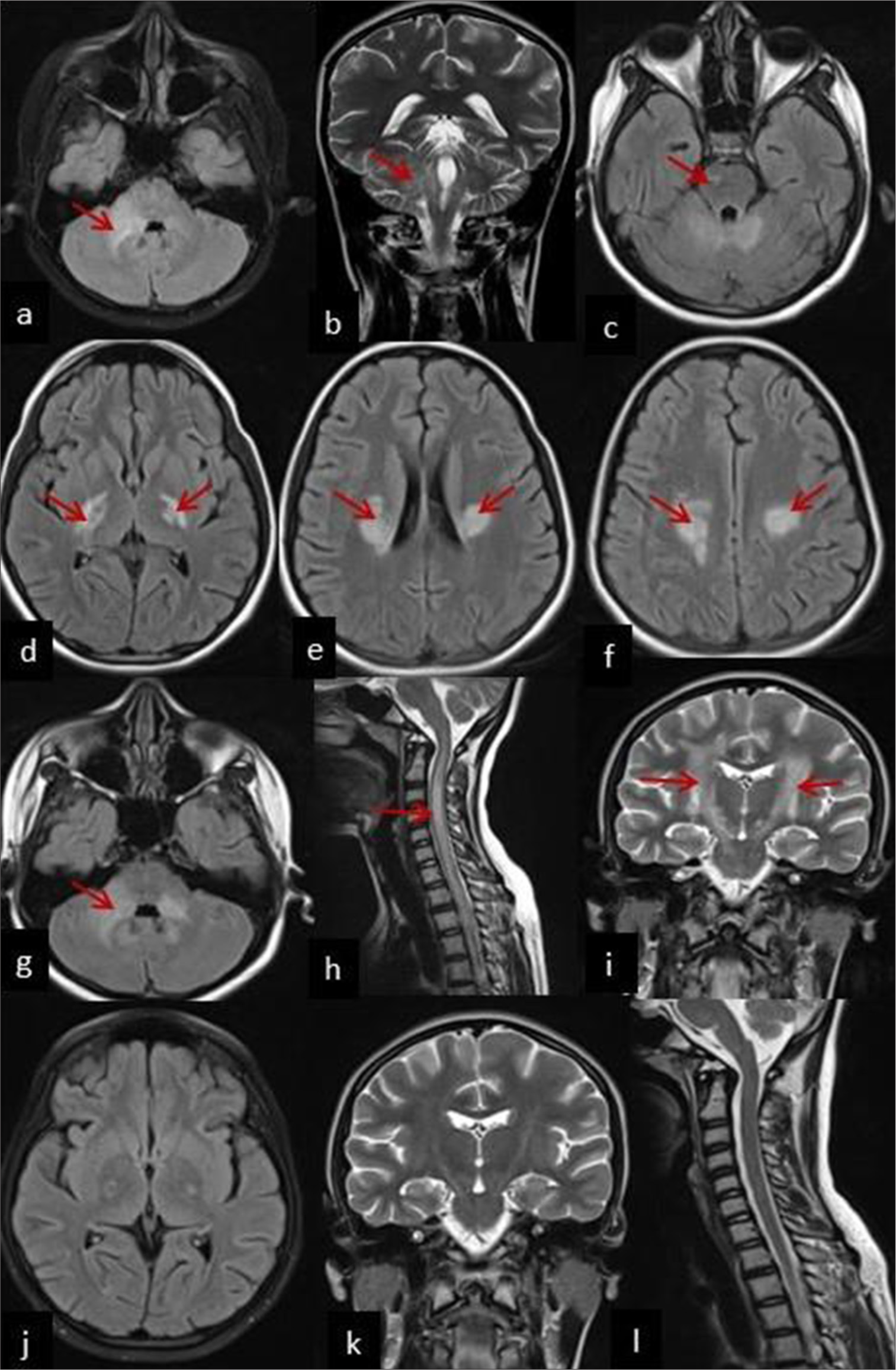
- Neuroimaging of Patient 4 can be divided into 3 stages-early stage, acute stage (after 10 days) and recovery stage. In early stage, (a) axial section T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in the right cerebellum (red arrow) and (b) coronal section T2/FLAIR hyperintensities in the right middle cerebellar peduncle (red arrow). Acute stage (After 10 days of onset): (c) Axial section T2/FLAIR hyperintensities in right pons (red arrow), (d) bilateral posterior limb of internal capsule (red arrow), (e) bilateral corona radiata (red arrow), (f) bilateral centrum semiovale (red arrow), (g) bilateral cerebellar peduncle (red arrow), and (h) cervical spine to cauda equina in spinal cord (red arrow). MRI brain coronal section showing (i) Tract sign-T2/FLAIR hyperintensities along the bilateral diffuse corticospinal tract (red arrow). Recovery phase (At three months follow-up post-treatment with Meropenem+TMP-SMX)-resolving T2/FLAIR hyperintensities along (j) bilateral corticospinal tract–axial section (k) and coronal section and (l) cervical spinal cord.
Patient 2
A 28-year-old male presented with a 10-day history of right-sided headache and earache, vomiting, hiccups, hoarseness of voice, and swaying to the right while walking. There was right horizontal monocular diplopia on all gaze, right 6–7th cranial nerve palsy, right hemiparesis, reduced touch and pain sensation on the right side of the body, and left cerebellar signs. Two days later due to worsening right hemiparesis, and new onset 9–10th cranial nerve palsy, he was electively intubated for airway protection. MRI brain findings are shown in Table 1. IHA was positive for B. pseudomallei. He received treatment for melioidosis, was weaned off the ventilator, and discharged.
Patient 3
A 23-year-old male presented with neck pain, headache, vertigo, and left eye double vision for three weeks. Right hemiparesis developed over 1 day. He had right-side gaze-evoked nystagmus on examination. By the 2nd day of admission, he developed left hemiparesis (Medical Research Council grade 2/5), and subsequently bilateral lower limb muscle power became 0/5. The patient exhibited hypertonia, brisk deep tendon reflexes, with extensor plantar response. Thus, he experienced acute onset rapidly progressive spastic quadriparesis with cerebellar involvement. He was electively put on mechanical ventilation to protect the airway. Given the clinical suspicion of demyelination, the patient had received a single dose of IV methylprednisolone. MRI brain revealed hyperintensities (T2/FLAIR) in (g) the left cerebellum, left middle cerebellar peduncle, (h) coronal section showed brainstem, (i) bilateral corticospinal tract and high frontoparietal region involvement [Figure 1] strongly suggestive of neurological melioidosis. He received treatment for melioidosis [Table 1], mechanical ventilation, and tracheostomy. The modified Rankin scale score at discharge was 5.
Patient 4
A 15-year-old girl presented with a two-week history of fever, headache, vomiting, and right-sided swaying for the past four days. CSF showed lymphocyte-predominant pleocytosis, and increased protein levels [Table 1], so initial early treatment included ceftriaxone and acyclovir for two weeks, resulting in the patient’s discharge. In the early stage, MRI brain showed T2/FLAIR hyperintensities in the (a) right cerebellum and in the (b) right middle cerebellar peduncle (denoted by red arrow) [Figure 2]. However, she returned after 10 days with paraparesis, sensory involvement at T6, bladder and bowel dysfunction, and brisk deep tendon reflexes. CSF analysis revealed a reduced cell count (70 cells) with lymphocyte predominance, elevated protein levels (113 mg/dL), and normal glucose. After 10 days of onset (Acute stage), neuroimaging findings (Axial section) are summarized in Table 1. MRI brain coronal section showed (i) tract sign-T2/FLAIR hyperintensities along the bilateral diffuse corticospinal tract [Figure 2].
Screening for abscesses elsewhere was negative. Antibody titers to B. pseudomallei were positive (1:2500) by IHA; thus, she was treated for melioidosis leading to improvement. Follow-up MRI brain taken at three months follow-up showed a recovery phase with resolving T2/FLAIR hyperintensities along (j) bilateral corticospinal tract – axial section, (k) coronal section, and (l) cervical spinal cord [Figure 2].
Patient 5
A 38-year-old diabetic male presented with a 10-day history of fever, headache, double vision, slurred speech, and imbalance while walking. Due to a decline in sensorium, the patient was intubated. Examination revealed right hemiparesis, reduced reflexes in the right upper limb, exaggerated right ankle jerk, and bilateral extensor plantar response. A prior evaluation elsewhere involved the administration of a single dose of pulse steroids suspecting demyelination. MRI brain findings are summarized in Table 1. Blood culture yielded B. pseudomallei and was treated for melioidosis and tracheostomized in view of prolonged mechanical ventilation.
DISCUSSION
Melioidosis presents with septicemic illness in Thailand and India,[1,2] whereas in Darwin northern Australia, the most common presentation is pneumonia either part of sepsis or resembling community-acquired pneumonia.[3] Neurological melioidosis is rare and occurred only in 14 of 540 (2.6%) cases in the 20-year Darwin study.[3] The manifestations of neurological melioidosis are varied. Originally, in 1992, the Darwin group described seven patients with neurological melioidosis, the manifestations of which were paraparesis mimicking Guillain-Barre syndrome, brainstem encephalitis, aseptic meningitis, and respiratory failure.[4] None of the available CSF samples grew the causative organism, thus leading to speculation that the neurological illness/syndrome was exotoxin-mediated. Next, they reported 12 cases where the presentation included monoparesis (n = 6), prominent cerebellar signs (n = 2), mixed cerebellar and brainstem features (n = 2), and paraparesis (n = 2).[5] The mortality was 25%.
This case series highlights the unusual presentation of isolated neurological melioidosis (B. pseudomallei), presented with diverse neurological symptoms such as spastic quadriparesis and cerebellar involvement in five patients without systemic sepsis and it can affect a wide age range (15–38 years). Early recognition of isolated neuromelioidosis and early treatment initiation can lead to favorable outcomes in most cases. It should not be overlooked or mistaken for demyelinating diseases and steroids should not be given. B. pseudomallei has a prolonged incubation period and microbiological confirmation is difficult. It is worthwhile to look for corticospinal tract hyperintensities tracking along the internal capsule (Tunnel sign), ring lesions, cerebellar microabscess, brainstem involvement, and trigeminal nerve root enhancement as they provide a strong clue to early diagnosis of neuromelioidosis as we reported in our five patients.[6] Neurological disease may also manifest as solitary, rapidly progressive, ring-enhancing lesions often with significant extra-axial involvement.[7,8]
Notably, the “Tunnel sign” – a linear or curvilinear enhancement along white matter tracts – was present in multiple patients of Burkholderia, strongly suggesting an infectious etiology, as it can also be seen in sparganosis (Spirometra mansoni) and Listeria.[8,9] The involvement of both the cerebellum, corticospinal tract and the brainstem in several cases further supports the predilection of B. pseudomallei for these regions.
The close differentials to be considered are Listeria rhombencephalitis, bacterial abscess, central nervous system tuberculosis, acute disseminated encephalomyelitis, and fungal infections.
CONCLUSION
Isolated neurological melioidosis, though rare, should be considered in patients presenting with subacute meningoencephalitis, multiple lower cranial nerve palsies, hemiparesis, cerebellar signs, and fever, particularly in endemic regions. This case series highlights the importance of neuroimaging in identifying neurological melioidosis as the presence of characteristic findings such as corticospinal tract hyperintensities (the “tunnel sign”), ring-enhancing lesions, and cerebellar or brainstem involvement helps in early diagnosis. Positive serological tests further support the diagnosis, enabling timely initiation of appropriate antibiotic therapy, such as meropenem, which significantly improves outcomes and reduces mortality. Misdiagnosis as a demyelinating diseases should be avoided, and the use of steroids without confirmation of the underlying etiology is discouraged. Early recognition and targeted treatment are essential for favorable patient outcomes in neurological melioidosis.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Clinical profile and predictors of mortality among patients with melioidosis. J Glob Infect Dis. 2023;15:72-8.
- [CrossRef] [PubMed] [Google Scholar]
- The Darwin prospective melioidosis study: A 30-year prospective, observational investigation. Lancet Infect Dis. 2021;21:1737-46.
- [CrossRef] [PubMed] [Google Scholar]
- Neurological melioidosis: Seven cases from the Northern territory of Australia. Clin Infect Dis. 1992;15:163-9.
- [CrossRef] [PubMed] [Google Scholar]
- Melioidosis: Insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2018;16:595-607.
- [Google Scholar]
- Melioidosis: Epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383-416.
- [CrossRef] [PubMed] [Google Scholar]
- Corticospinal tract involvement in MRI of neuromelioidosis: Report of three cases with a review of clinicoradiological features. Neurol India. 2022;70:767-71.
- [CrossRef] [PubMed] [Google Scholar]
- Spreading of multiple Listeria monocytogenes abscesses via central nervous system fiber tracts: Case report. J Neurosurg. 2015;123:1593-9.
- [CrossRef] [PubMed] [Google Scholar]






