Translate this page into:
Cerebral tuberculoma as a manifestation of paradoxical reaction in patients with pulmonary and extrapulmonary tuberculosis
Address for correspondence: Dr. Anirban Das, Peon Para, Bhatchala, P.O. Sripalli, Burdwan – 700 103, West Bengal, India. E-mail: dranirbandas_chest@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Expansion of cerebral tuberculomas or their new appearance as a manifestation of paradoxical reaction in patients under antituberculous chemotherapy is well documented. Distinguishing paradoxical reaction from disease progression or treatment failure is an important issue in tuberculosis management. Five cases of cerebral tuberculomas are reported here as manifestations of paradoxical reaction in patients with pulmonary and extrapulmonary tuberculosis on antituberculous treatment. Case 1 and 2 had tuberculous meningitis, Case 3 had miliary tuberculosis, Case 4 had miliary tuberculosis and destructive vertebral lesions, and Case 5 had pulmonary tuberculosis. Continuation of antituberculous drugs and addition of steroids led to full recovery of all patients.
Keywords
Intracranial
paradoxical reaction
tuberculoma
tuberculosis
Introduction
Paradoxical reactions (PRs) are defined as transient worsening or appearance of new signs or symptoms or radiographic manifestations of tuberculosis (TB) that occur after initiation of treatment, and are not the result of treatment failure or a second process.[1] Generally, the patients have received anti-tuberculosis treatment for at least 2 weeks and have been improving initially. The time to onset of PR is defined as the number of days from the start of treatment to the commencement of deterioration.[2] PR during the course of therapy with antituberculous drugs (ATD) is most frequently observed in lymph nodes, but is also encountered in brain and lungs.[3] Intracranial tuberculomas develop in approximately 1% of all patients with active TB[4] and 4.5–28% of those with tuberculous meningitis.[5] The authors report five cases of pulmonary and extrapulmonary TB who developed PR manifested as symptomatic cerebral tuberculomas during the course of their illnesses and discuss the features of this neurologic localization. PR in the form of cerebral tuberculoma commonly occurs in patients with tuberculous meningitis, but it is also seen in patients of miliary TB and pulmonary TB. We are reporting this case series to increase the awareness among clinicians regarding consideration of PR as a cause of transient clinico-radiological deterioration in patients with TB, its possible differential diagnosis, and the best possible management.
Case Reports
Tuberculomas complicating the treatment of TB meningitis
Case 1
A hypertensive, non-diabetic male of 35 years presented with irregular high fever and altered sensorium for more than 24 h with history of low-grade continuous fever for the last 2 months. Total lymphocyte count in peripheral blood was 4500/μl. Cerebrospinal fluid (CSF) analysis showed cell count 30/μl with 80% lymphocytes, glucose 20 mg/dl, protein 184 mg/dl, and adenosine deaminase (ADA) 87 U/l; DNA polymerase chain reaction (PCR) of Mycobacterium tuberculosis was positive. No acid-fast bacillus (AFB) was detected in the CSF smear and pyogenic and mycobacterial cultures of CSF were negative. Contrast-enhanced CT (CECT) scan of brain revealed no abnormality. HIV serology was non-reactive. Chest X-ray was normal. He was put on WHO category-I ATD (isoniazid, rifampicin, pyrazinamide, ethambutol) along with oral prednisolone 30 mg daily as a case of tuberculous meningitis and the evolution was initially favorable. But after 1 month, he was readmitted with neck pain and headache, followed by weakness of both lower limbs and retention of urine. Clinical examination revealed exaggerated knee and ankle jerks with decreased tone and power of lower limbs of both sides in addition to bilateral extensor plantar responses. Total lymphocyte count in peripheral blood was 6000/ μl. Magnetic resonance imaging (MRI) of brain revealed multiple inflammatory granulomas in both cerebral hemispheres, associated with focal meningitis in right temporal region and lacunar infarct in pons. MRI of dorsal spine revealed tuberculous inflammatory lesion and spinal cord compression with edema at D3–D4 level. MRI myelogram revealed anterior displacement of spinal cord at D1–D8 level with no intrathecal contrast above D1 level, suggesting complete block [Figure 1]. ATD and oral prednisolone 40 mg daily were continued along with addition of phenytoin. Prednisolone was continued for 1 month, followed by gradual tapering over the next 6 weeks. Surgical intervention was retained, but the patient refused it. However, he gradually improved after 2 weeks of increment of steroid dose, but had residual spastic paraparesis at the end of 9 months of chemotherapy.
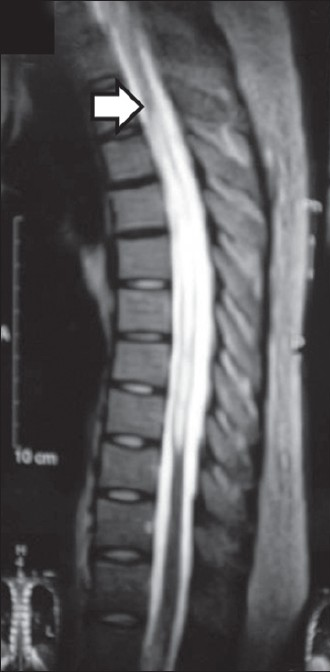
- MRI myelogram revealing anterior displacement of spinal cord at D1–D8 level with no intrathecal contrast above D1 level (Case 1)
Case 2
A 5-year-old male child was admitted with history of low-grade fever, irritability, and headache for 4 months. Total lymphocyte count in peripheral blood was 3000/μl. Chest radiograph showed fibrotic lesion in right upper zone. Sputum smear for AFB and culture for M. tuberculosis were negative. CSF examination showed 240 cells/μl, 90% lymphocytes, glucose 38 mg/dl, protein 223 mg/dl, and ADA 30 U/l. No AFB was detected in the CSF smear, and pyogenic and mycobacterial cultures of CSF were negative. DNA PCR of M. tuberculosis was positive. CT brain showed effacement with enhancement of basal cisterns and Sylvian fissures with moderately dilated supratentorial ventricles suggestive of tuberculous meningitis. A dense calcified left cerebellar lesion was also seen. The patient was prescribed WHO category-II ATD (isoniazid, rifampicin, pyrazinamide, ethambutol, streptomycin), as he had previous history of pulmonary TB 2 years back. Forty milligrams oral prednisolone was given for 1 month, followed by gradual tapering over 6 weeks. The patient was improving till he was readmitted after 4 months with history of involuntary movements in right side of the body involving both the limbs for 4 days. Total lymphocyte count in peripheral blood was 5500/ μl. CECT brain showed multiple ring-enhancing lesions in both cerebral hemispheres, in addition to the calcified lesion in the left cerebellum, identical to the initial one. MRI of brain showed multiple granulomatous lesions suggestive of tuberculomas in the brainstem causing obstructive hydrocephalus. A large calcified granuloma was also noted in left cerebellar region. HIV serology was nonreactive. The patient was prescribed oral sodium valproate and injection dexamethasone along with continuation of ATD and he improved gradually within 4 weeks. Four milligrams dexamethasone was given intravenously eight hourly for 1 month, followed by gradual tapering with oral prednisolone over the next 6 weeks. Total duration of ATD was 9 months. He was left with no neurodeficit at the end of therapy.
Tuberculomas complicating the treatment of miliary TB
Case 3
A 22-year-old female presented with high-grade intermittent fever with dry cough for 2 months. Total lymphocyte count in peripheral blood was 4000/μl. Chest X-ray revealed miliary mottling. Ultrasonography (USG) of whole abdomen revealed splenomegaly, and enlarged lymph nodes in porta hepatis and peripancreatic region. Sputum smear for AFB and culture for M. tuberculosis were negative. WHO category-I ATD (isoniazid, rifampicin, pyrazinamide, ethambutol) was started as a case of miliary TB. The evolution was marked by the development after 1 month of severe persistent holocranial headache, vomiting, photophobia, and pain with redness of the left eye. Examination of nervous system was normal, as it was the case of the analysis of liver function, CSF, and total lymphocyte count in peripheral blood. HIV serology was nonreactive. CECT scan of brain revealed multiple small nodular and ring-enhancing lesions with edema in both cerebral hemispheres that are suggestive of tuberculomas [Figure 2]. Injection dexamethasone and tablet lamotrigine were added. Four milligrams dexamethasone was given intravenously eight hourly for 1 month, followed by gradual tapering with oral prednisolone over the next 6 weeks. The patient clinically improved within 1 month. After 9 months of ATD, both thoracic and intracranial lesions radiologically disappeared and no residual neurodeficit was detected.
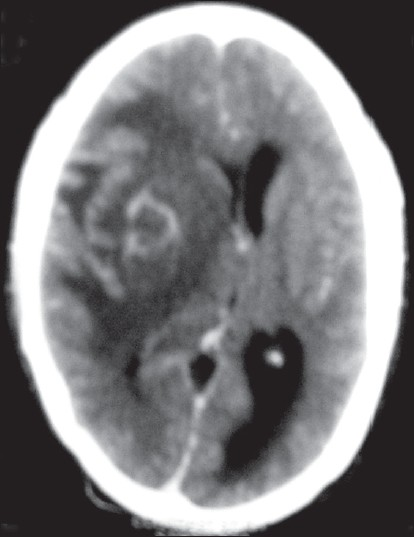
- CECT brain showing multiple ring-enhancing lesions with edema in both cerebral hemispheres (Case 3)
Case 4
A 10-year-old female presented with history of cough, fever, and weight loss for 3 months, and pain in the back for 2 months. Total lymphocyte count in peripheral blood was 3000/μl. Chest X-ray revealed miliary mottling. X-ray of dorsolumbar spine revealed destructive vertebral lesions at upper lumber region (L2–L3). Sputum smear for AFB and culture for M. tuberculosis were negative. She was put on WHO category-I ATD (isoniazid, rifampicin, pyrazinamide, ethambutol). She was improving with treatment, but was re-admitted after 2 months with appearance of generalized tonic clonic seizures. Total lymphocyte count in peripheral blood was 4500/μl. CSF analysis showed 400 cells/μl with 80% lymphocytes. CECT scan of brain showed conglomerated nodular lesions suggestive of tuberculomas involving right temporal area with perilesional edema. HIV serology was nonreactive. The patient was given oral phenytoin and 40 mg prednisolone daily with continuation of ATD. Prednisolone was continued for 1 month, followed by gradual tapering over the next 6 weeks. She improved within 2 weeks and successfully completed 9 months of chemotherapy without any residual lesion in brain or spine.
Tuberculomas complicating the treatment of pulmonary TB
Case 5
A 30-year-old male patient presented with history of productive cough, low-grade fever, and occasional hemoptysis for 1 month. Clinical examination and investigations revealed pneumonic opacities in left lower lobe. Total lymphocyte count in peripheral blood was 5500/μl. Sputum smear was positive for AFB. Sputum culture showed growth of M. tuberculosis, sensitive to all five first-line ATDs. As the patient had previous history of pulmonary TB 4 years back, WHO category-II ATD (isoniazid, rifampicin, pyrazinamide, ethambutol, and streptomycin) was started as a case of relapse pulmonary TB. He was improving with the treatment.
The evolution was marked after 3 months of chemotherapy by the appearance of headache, vomiting, and left upper limb weakness for 5 days. Neurological examination revealed grade II/V weakness in the left upper limb with no sensory involvement. He was disoriented and drowsy during admission and ophthalmoscopy revealed papilledema. Total lymphocyte count in peripheral blood was 6000/μl. CSF study showed 100 cells/μl with 90% lymphocytes. CT brain showed multiple tuberculomas in the brain with gross perilesional edema in both cerebral hemispheres. HIV serology was nonreactive. The patient was treated with injection dexamethasone and ATD was continued. Four milligrams dexamethasone was given intravenously eight hourly for 1 month, followed by gradual tapering with oral prednisolone over the next 6 weeks. The patient clinically improved within 1 month and he received 9 months of antituberculous chemotherapy. At the end of treatment, he had complete functional recovery.
Discussion
The phenomenon of PR is frequently observed in TB patients co-infected with HIV after the commencement of Highly Active Antiretroviral Therapy (HAART) where patients experience temporary exacerbation or worsening of symptoms, signs, and/or radiographic manifestations of TB disease.[6] But such phenomenon is also described in TB patients without HIV infection after the starting of ATD in 2–23%;[7] however, the frequency of PR is much lower in these groups compared with patients receiving HAART.[8] It manifests clinically as worsening of fever, cough, chest infiltrates, or increased lymphadenopathy or symptomatic tuberculoma. A tuberculoma results when intracranial tubercles enlarge without rupturing into the subarachnoid space. Development of symptomatic intracranial tuberculomas while on therapy was first described in 1974 by Thrush and Barwick.[9] When tuberculomas occur as PR, original sites of infections could be pulmonary, meningeal, or miliary.[10] The latent period between start of chemotherapy and development of intracranial tuberculoma is between 10 days and 5 months,[10] but it may be delayed up to 18 months.[11] Most adult patients present with seizures, while constitutional symptoms are less. Focal weakness and papilledema are most frequent signs. Sizes of the tuberculomas vary from a few millimeters to 4 cm. They are found in cerebral hemisphere, basal ganglia, brain stem, cerebellum, and spinal cord. They are usually infratentorial in children and supratentorial in adults. Solitary lesions are more frequent than multiple lesions. The CT and MRI have revolutionized the diagnosis of intracranial tuberculomas. The characteristic CT and MRI findings are nodular enhancing lesions with a central hypointensity. The enhancement may be homogenous, patchy, serpentine, or ring enhancing. Perilesional edema is often marked. Similar CT findings are also seen in glioma, metastasis, abscess, neurocysticercosis, and fungal granuloma. Angiographically tuberculoma appears as an avascular mass, sometimes with a faint tumor blush. Demonstration of caseating granuloma or AFB in stereotactic biopsy of the lesion is gold standard for the diagnosis.
Adjunctive corticosteroid treatment improves the symptoms and seizure control and reduces tuberculoma size and perilesional edema radiographically. Thalidomide has been used successfully in a few patients to reduce the size of tuberculomas.[12] Similarly, infliximab, a tumor necrosis factor-α blocker, was used to control severe paradoxical reaction of brain and lymph nodes.[13] Surgical therapy for intracranial tuberculoma is usually considered only when medical therapy fails, when decompression is necessary, or when the diagnosis is uncertain. The outcome of most patients is favorable, but few cases may end with residual disability, even death.[14] The cause of the PR in immunocompetent person is not yet known, but is supposed to be having immunological basis. The pathogenesis has been attributable to several factors like persistence of lipid-rich insoluble cell wall antigen infected tissue, exposure and release of new antigen targets during mycobacterial killing, hypersensitivity to tuberculoprotein, and exaggerated immune restoration (following TB-induced immunosuppression) occurring on TB treatment.[715]
In the present study, there were five patients, all immunocompetent and compliant with their antituberculous therapy. One patient was suffering from miliary TB, one patient from miliary TB with destructive vertebral lesions, two patients from TB meningitis, and one patient from pulmonary TB. They all developed PR mainly in the central nervous system after commencement of ATD. The latent period between commencement of ATD and development of PR was between 1 and 4 months. PR may involve the initial site of the disease manifestation, but sometimes may appear in distant site which may lead to diagnostic confusion. It is important to recognize these clinically frustrating but benign PR, which usually does not require change in therapy. We should be aware of these events and at the same time consider other causes of inadequate response such as wrong diagnosis, inadequate drug regimen, multi-drug resistant TB, atypical mycobacterial disease, complication of drug therapy, or progression of the disease itself before attributing their signs and symptoms to PR.[16] So, a high index of suspicion is needed for the diagnosis of PR. In fact, an initial improvement followed by deterioration despite firm diagnosis and adequate therapy are strong evidence to suspect PR on clinical grounds. These reactions can be effectively managed with continuation of ATD. Systemic corticosteroid is probably indicated for most of the neurological PR to manage symptoms and cerebral edema.[17] This is well evident from the present study, as all our patients improved with the above treatment.
Source of Support: Nil
Conflict of Interest: None declared
References
- Treatment of HIV-related tuberculosis in the era of effective antiretroviral therapy. Am J Respir Crit Care Med. 2001;164:7-12.
- [Google Scholar]
- Epidemiological, therapeutic and evolutionary profiles in patients with lymph node tuberculosis. Tuberk Toraks. 2010;58:366-74.
- [Google Scholar]
- Paradoxical responses during the chemotherapy of tuberculosis. J Infect. 1987;15:1-3.
- [Google Scholar]
- Atypical response to chemotherapy in neurotuberculosis. Br J Neurosurg. 1998;12:344-8.
- [Google Scholar]
- Intracranial tuberculoma developing during therapy for tuberculous meningitis. West J Med. 1990;152:188-90.
- [Google Scholar]
- Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361-73.
- [Google Scholar]
- Risk factors for d evelopment of paradoxical response during antituberculosis therapy in HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2003;22:597-602.
- [Google Scholar]
- Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59:704-7.
- [Google Scholar]
- Three patients with intracranial tuberculomas with unusual features. J Neurol Neurosurg Psychiatry. 1974;37:566-9.
- [Google Scholar]
- Symptomatic intracranial tuberculoma developing during treatment of tuberculosis: A report of 10 patients and review of the literature. Q J Med. 1987;63:449-60.
- [Google Scholar]
- Intracranial tuberculomas: Correlation of computerized tomography with clinico-pathological findings. QJ Med. 1982;51:104-14.
- [Google Scholar]
- The use of thalidomide in the treatment of intracranial tuberculomas in adults: Two case reports. J Infect. 2003;47:251-5.
- [Google Scholar]
- Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47:e83-5.
- [Google Scholar]
- Paradoxical enlargement or development of intracranial tuberculomas during therapy: Case report and review. Clin Infect Dis. 1994;19:1092-9.
- [Google Scholar]
- Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009;30:797-810.
- [Google Scholar]
- Splenic abscess as a paradoxical response to chemotherapy in tuberculous pleural effusion. Ann Thorac Med. 2010;5:50-1.
- [Google Scholar]
- Paradoxical Reactions and the Immune reconstitution inflammatory syndrome. In: Schlossberg D, ed. Tuberculosis & Nontuberculous My cobacterial Infections (5th ed). New Delhi: Tata McGraw-Hill; 2007. p. :400-11.
- [Google Scholar]






