Translate this page into:
Cerebellar infarct with neurogenic pulmonary edema following viper bite
Address for correspondence: Dr. Salil Gupta, Department of Neurology, Armed Forces Medical College, Sholapur Road, Pune – 411 040, India. E-mail: chickusalil@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Russell's viper (Daboia russelli) bites are well known to cause bleeding complications. However, thrombotic complications are rare. We present the case details of a female who was bitten by a Russell's viper (Daboia russelli) in her village. She then developed features of envenomation in the form of hemorrhagic episodes. She received 27 vials of polyvalent anti-snake venom to which the hemorrhagic complications responded. After about 48 h of the bite she developed features of cerebellar infarct along with pulmonary edema which was in all probability neurogenic in origin. She was managed with mechanical ventilation and extra ventricular drainage with good recovery. We discuss the likely pathogenesis of the infarct and pulmonary edema occurring in a patient with viper bite and other features of envenomation.
Keywords
Cerebellar infarct
neurogenic pulmonary edema
viper bitee
Introduction
Envenomation by the bite of Russell's viper (Daboia russellii) is common in rural India. The bite causes severe local necrosis and systemic complications chiefly related to coagulation derangement. The coagulation derangements are usually in the form of bleeding manifestations. Distant thrombosis is extremely rare. There are only a few case reports in the literature of viper bite leading to ischemic stroke.[1–6] Most documented case reports are of anterior circulation ischemic stroke except one.[7] We report a cerebellar stroke associated with neurogenic pulmonary edema following the bite of a viper.
Case Report
Our patient, a 48-year-old lady from the state of Karnataka, India, had no known co-morbidities. She was a home maker. One evening, while cleaning the courtyard of her house in her village she was bitten by a snake on her right ankle. The snake was killed by her family members and identified by locals as Russell's viper (Daboia russellii). She was taken to the local district hospital which was about 2 h from her village. On admission, the receiving medical officer's notes documented her to have significant swelling around her right ankle extending up to her leg. Rest of the clinical examination was normal. She had no neurological manifestation. Within 3 h from the time of the bite, she developed hematemesis and an hour later had hematuria also. When tested her blood did not clot but her other biochemical parameters including renal function tests were normal. Coagulation profile was not available in that hospital. She was started on reconstituted polyvalent anti-snake venom (10 ml per vial) around 4 ½ to 5 h after the bite. A total of 27 vials of anti-snake venom were given over 48 h. The clinical end point used was clearing of urine. On the third morning after the snakebite she was noticed to be drowsy with breathing difficulty. Due to the respiratory distress she was intubated. Since there were no neurological facilities available at the local hospital and she was entitled free treatment in armed forces hospital, she was shifted to our tertiary care center which is located about 200 km from the district hospital. She was shifted by road and reached our center around 6-7 h after the onset of drowsiness.
At admission, she was in respiratory distress and required ventilatory support. Pulse was 124/min and BP was 90/60 mm of Hg. Chest had bilateral crepts. Neurologically she was drowsy but obeying commands. Glasgow Coma Scale was E3M6Vt. The verbal response could not be assessed since she had an endotracheal tube in place. Left pupil was a little larger than right with sluggish reaction to light. Skew deviation of left eye with nystagmus on looking to left were present. She was moving left side less as compared to the right. All reflexes were present with left plantar being extensor. Cellulitis with local skin necrosis at the site of bite with swelling of right leg was also present.
Investigations were as follows: Hb 9.3 g/dl, TLC 25000/mm3, Polymorphs 89%, platelet count 80000/mm3, prothrombin time (PT) was test 13 s control 17s, INR 1.4, APTT control 27 s and test 36 s, d dimmer assay 2.1 mg/dl (normal 0-0.3 mg/dl), fibrinogen degradation products > 20 μg/dl (raised), urine routine examination: 5-6 pus cells and 8-10 RBCs/HPF. Chemistries including renal function tests, liver function tests, and electrolytes were all normal. Chest X-ray [Figure 1] showed features of pulmonary edema and plain CT brain showed left cerebellar infarct with mass effect, distortion of the fourth ventricle, dilatation of the lateral and third ventricle with periventricular ooze. ECG, 2D echocardiography, carotid artery and lower limb venous Doppler studies were all normal.
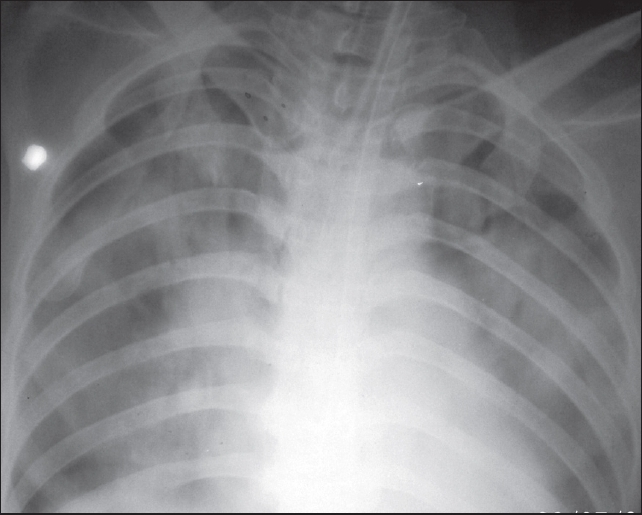
- Bedside X-ray chest showing bilateral fluffy opacities suggestive of pulmonary edema
In view of the obstructive hydrocephalus, an external ventricular drainage (EVD) was placed to decompress the ventricles [Figure 2]. Raised intra cranial pressure (ICP) was further managed with 30° head up position and decongestive measures including diuretics and mannitol. Intermittent hyperventilation to maintain CO2 at around 25 mmHg was also done. Antiplatelets and low-molecular-weight heparin in prophylactic doses to prevent DVT were also given. Antibiotics including vancomycin 1g IV q12h, ceftriaxone 2g IV q12h, and metronidazole 500 IV q8h were given for the cellulitis.
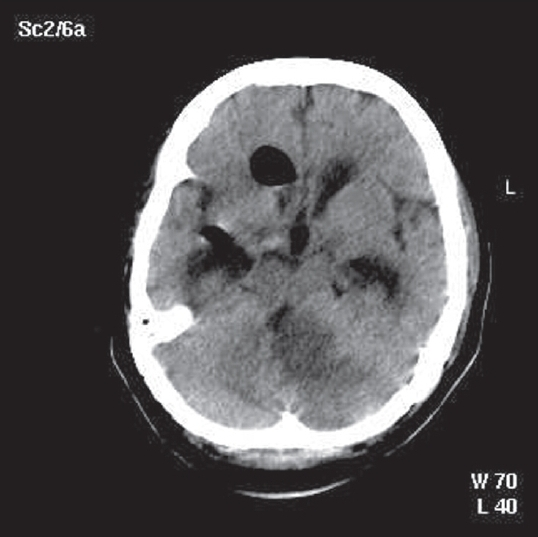
- Post EVD non contrast CT brain showing left-sided cerebellar infarct with a mass effect pushing the brainstem to the right, chinking of fourth ventricle and hydrocephalus. Also seen is air within the ventricle following the EVD
She gradually improved with this therapy and could be weaned off on day 5 after being placed on the ventilator. The local cellulitis healed. Since she showed neurological improvement with ICP settling down EVD could be removed safely on day 7 post stroke. Subsequently, a four vessel DSA of the brain vasculature was normal. She was gradually ambulated and at discharge she could walk with support. She was advised physiotherapy and to continue aspirin. On follow up over the last 7 months, she has regained complete ambulation and does all activities of daily living on her own. We have continued her on aspirin 150 mg/ day.
Discussion
The usual clinical manifestations following a Russell's viper (Daboia russelli) bite depend on the severity of envenomation. Severe envenomation may lead to pain, swelling, and blister formation locally. Systemic manifestations include abdominal pain, nausea, vomiting, severe hypotension, tachypnea, dyspnea, tachycardia, and neurologic signs and symptoms. Bleeding is a major complication, either from the bite site or from mucosal surfaces. Hemorrhagins and hemolysins act on the vessel wall destroying them and along with coagulation defects lead to blood loss severe enough to require a transfusion.
In addition to anticoagulant effects, viper venoms also have procoagulant effects.[2] The coagulant effect is likely due to arginine esterase hydrolase, an enzyme that is similar in action to thrombin, and which clots fibrinogen and aggregates platelets. The coagulant effects may also be due to the conversion of prothrombin to thrombin, a change catalyzed by proteinases. This triggering of the coagulation cascade in vivo results in the formation of microthrombi, the activation of fibrinolysis, and a bleeding tendency, which could lead to hemorrhagic complications.
Mosquera et al. reported cerebrovascular complications in 8 patients out of 309 in their series of snakebites (2.6%). Seven of these had hemorrhagic strokes and one person had ischemic stroke.[8] Ischemic strokes following viper bite, although rare, have been documented earlier. Most cases of have been reported from Asia especially South East Asia.[1–5] These ischemic events have occurred a few hours after envenomation unlike our patient who developed a cerebellar infarct after 48 h. Bashir et al reported a 13-year-old girl who developed a right hemiparesis with aphasia 10 h after envenomation[1] while Panicker et al reported the event within 2 h of the bite.[4] However, the cause of cerebral infarction in these reports was not clear. A possible explanation given was that the cerebral infarction could be related to the vessel-damaging toxin in the venom, possibly acting on a pre-existing abnormality in the blood vessel wall. Other possibilities they considered included low-grade dissemination of intravascular coagulopathy and hypotension.[2]
To our knowledge this is the first case of cerebellar ischemic stroke accompanied by neurogenic pulmonary edema following a viper bite although a brainstem infarct has been reported earlier by Lee et al and cerebellar infarct by Mugundhan et al.[79]
The possible explanation for the ischemic infarct in our patient too could be related to microthrombi as a part of consumptive coagulopathy since this was documented by laboratory findings. Other reasons could be venom damaging the endothelial lining of vessel wall leading to thrombosis, venom acting on a pre-existing damaged segment of vessel wall producing thrombosis or occult hypotension although the infarct was not in a watershed territory. The subsequent four-vessel angiography proved that there were no pre-existing vascular abnormalities and also that the present problem reversed.
The patient developed pulmonary edema in the absence of any cardiac disease. This non-cardiogenic pulmonary edema developed temporally in relation to the time when her intracranial pressure was raised due to the cerebellar infarct. Raised intracranial pressure, especially a rapid rise due to a posterior fossa event, may give rise to a burst of autonomic discharge which may raise the hydrostatic pressure in the pulmonary circulation giving rise to pulmonary edema. Other possible explanation for the non-cardigenic pulmonary edema in this patient may be related to vascular endothelial injury due to circulating toxin of the venom or as a part of acute lung injury due to sepsis. However, since the onset of the pulmonary edema was at the same time as the cerebellar infarct with raised intracranial pressure, it is likely to be neurogenic in origin. The pulmonary edema also responded to bringing down the raised intracranial pressure.
Our patient has recovered well after the acute insult. Although on she has no identifiable vascular risk factor and the four-vessel DSA of the brain is normal, we have chosen to continue the antiplatelet drug in view of the possibility of occult damage to the endothelium which may have led to the stroke.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Cerebral infraction after envenomation by viper. J Comput Assist Tomogr. 1997;21:35-7.
- [Google Scholar]
- Middle cerebral occlusion following Russel's viper bite. J Trop Med Hyg. 1972;76:95-7.
- [Google Scholar]
- Cerebral infarction in a young male following viper envenomation. J Assoc Physicians India. 2000;48:744-5.
- [Google Scholar]
- Acute ischemic infarct in the middle cerebral artery territory following a Russell's viper bite. Neurol India. 2009;57:479-80.
- [Google Scholar]
- Bilateral anterior cerebral artery infarction following viper bite. J Assoc Physicians India. 2009;57:67-9.
- [Google Scholar]
- Posterior circulation stroke in a young male following snakebite. J Assoc Physicians India. 2008;56:713-4.
- [Google Scholar]






