Translate this page into:
Butterfly Tumor of the Corpus Callosum: Clinical Characteristics, Diagnosis, and Survival Analysis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The pathologies implicate the bilateral corpus callosum that builds the butterfly pattern on axial view. These tumors have seldom been investigated for both clinical manifestations and outcome.
Objective:
The objective of this study was to describe the clinical characteristics and outcomes of the butterfly tumor and to identify the predictive factors associated with survival outcome.
Methods:
A retrospective study of 50 butterfly tumor was conducted between 2003 and 2016. The clinical characteristics, imaging, and outcome were assessed for the purpose of descriptive analysis. Using the Kaplan–Meier method, the median overall survival of the butterfly tumor was determined. Furthermore, the Cox proportional hazard regression was the estimated hazard ratio for death.
Results:
Diffuse large B-cell lymphoma was common of butterfly lesions. The mortality rate was 78% and overall median survival time was 16.03 months (95% confidence interval: 14.0–19.8). Using Cox proportional hazards regression, the independent prognostic factors were Karnofsky Performance Status score ≤70, splenium involvement, and butterfly glioblastoma.
Conclusions:
The butterfly tumor is a poor prognostic disease compared with each histology subgroup. Further molecular investigation is preferable to explore genetic variations associated with these tumors.
Keywords
Butterfly glioma
corpus callosum glioblastoma
glioblastoma
primary central nervous system lymphoma
INTRODUCTION
The corpus callosum is the interhemispheric commissure. The fibers from the inferior frontal and anterior inferior parietal lobe cross in the genu. The fibers from the parietal lobe cross at the splenium. Therefore, the remaining fibers cross at the body of the corpus callosum.[1] The pathologies of the corpus callosum are various, including congenital malformation, demyelination, infectious diseases, traumatic lesions, and neoplasms. The pathological lesions primarily involve the corpus callosum; thus, these create bilateral hemispheric patterns results in a butterfly pattern.[2] Glioblastoma and primary central nervous system lymphoma (PCNL) have been mentioned as common histopathologies of the butterfly tumor.[34] Furthermore, extramedullary myeloid sarcoma (granulocytic sacroma),[5] toxoplasmosis,[6] and neuronal ceroidlipofuscinosis (Kufs disease) have been recently reported.[7]
From the literature, few studies mention this specific lesion. In Dziurzynski et al.'s study of butterfly glioblastomas, the prevalence was 2.9% of all cases and the median overall survival was 6 months.[3] In Dalia et al.'s study of PCNL, the median overall survival was 35 months while higher Eastern Cooperative Oncology Group performance status (score 2–4) was correlated with a worse prognosis.[8] In tumor conditions, the treatment of butterfly neoplasms is really challenging because surgical management is limited to extensive resection. At present, no defined optimal treatment or practical management includes biopsy followed by radiation and chemotherapy. Due to data limitations, we described the clinical characteristics, histological diagnosis, outcome, and survival of the bihemispheric lesion as our primary goal. Our secondary goal was the exploration of factors associated with survival time.
METHODS
The study was performed with the permission of the Ethics Committee of the Faculty of Medicine, Songklanagarind Hospital, Prince of Songkla University.
We searched patients treated in Songklanakarind Hospital based on the hospital information system (HIS). The inclusion criteria were comprised patients who underwent surgery between 2003 and 2016, neuroimaging that showed butterfly tumor at the corpus callosum, and histological diagnosis being available. The subdivision of the corpus callosum was assessed on the sagittal plane adapted from Highley et al.[9] [Figure 1a]. The butterfly lesion was defined as an intracranial lesion involving bilateral corpus callosum [Figure 1b–e]. Fifty-one patients matched our criteria; however, 1 patient was excluded because their medical records were inaccessible. Finally, the total population in the present study was 50 patients.
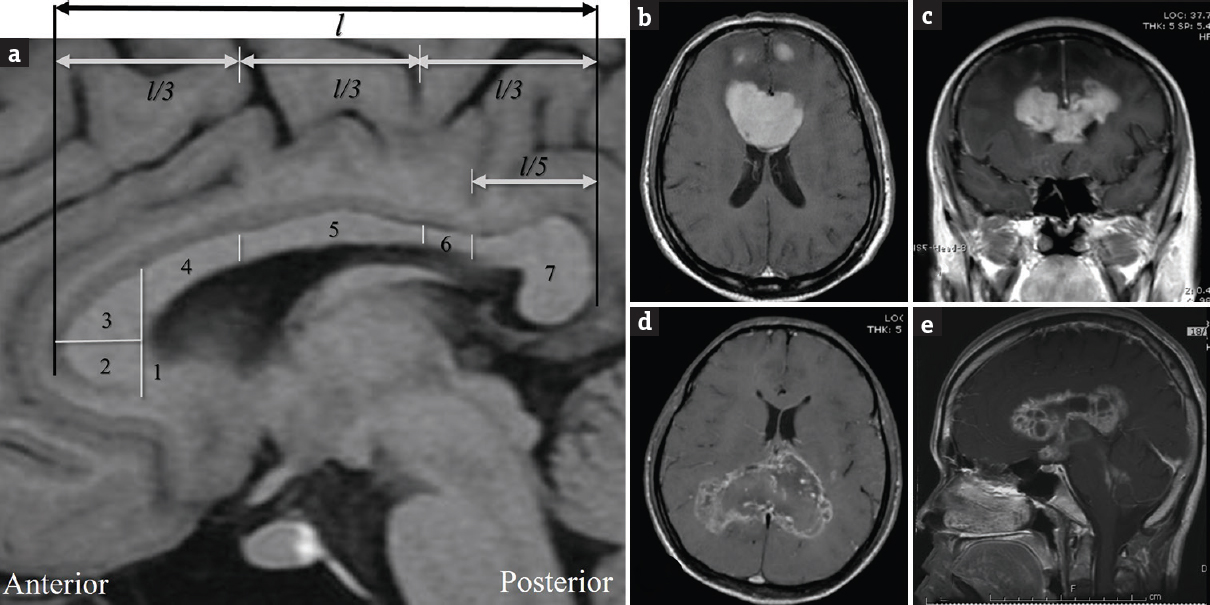
- (a) The length of corpus callosum (l) is divided on the sagittal plane including (1) rostrum, (2) inferior genu, (3) superior genu, (4) posterior genu, (5) body, (6) isthmus, and (7) splenium in T1-weighted magnetic resonance imaging that adapted from Highley et al. (b and c) The contrast-enhanced T1-weighted magnetic resonance imaging showing vivid enhancement of genu lymphoma with bifrontal extension. (d) The axial contrast-enhanced T1-weighted magnetic resonance imaging showing low enhancement of splenium glioblastoma. (e) The sagittal contrast-enhanced T1-weighted magnetic resonance imaging showing enhancement of whole corpus callosum germinoma with subependymal dissemination
The Karnofsky Performance Status (KPS) score is an assessment tool for functional impairment. Scores run from 100 to 0. In most serious illnesses, the lower the KPS score, the worse the likelihood of survival. KPS scores were dichotomized into two groups, according to ability to carry on normal activities (KPS score >70).[10] Therapeutic factors, including surgical resection, type of surgery, and adjuvant therapy, were determined. In the present study, enrollment date means the first surgical date. Therefore, the living status of patients was evaluated using medical records from when the patients died at the hospital and phone interviews or local municipality records if patient deaths were not recorded in the HIS.
Neuroimages were retrospectively assessed for the lesion configurations, such as tumor location, degree of edema, necrosis, and hypervascularization. The hypervascularization of tumor was defined as the visualizing vascular structures inside or around a tumor (flow void sign) in neuroimaging.[11] Meanwhile, the degree of mass effect, tumor necrosis, and enhancement were determined according to Lacroix et al.[12]
Statistical analysis
Patient characteristics, imaging factors, and therapeutic factors were analyzed using descriptive analysis presented as proportions, mean ± standard deviation (SD). For survival analysis, the median overall survival time was determined. Therefore, each factor was evaluated using log-rank tests and Kaplan–Meier's survival curve was constructed. The Cox proportional hazard regression model was used to analyze predictors of survival. A P < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 17.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Clinical characteristics
The clinical manifestations of the population are shown in Table 1. The butterfly tumors were predominately found in males and were common in adults. Therefore, the mean age was 48.94 (+SD 16.3) while ranging in age between 11 and 84 years. The patients usually suffered progressive headaches and hemiparesis. Less than 20% of cases had seizures at the presentation. Most of the patients had an unfavorable performance status. Most glioma was found in adults with a mean age of 48.1 years ± SD 15.3. There was one case of a 13-year-old boy with diffuse astrocytoma. Moreover, all of PCNL was in the adult population, with a mean age of 55.5 years ± SD 11.9.
The diffuse large B-cell lymphoma with non-AIDS was the most common diagnosis while glioblastoma could be found in 30% of cases. Interestingly, the germ cell tumor was rarely diagnosed (6%). Based on anatomical configuration, the tumors immersed frequently at the genu of corpus callosum while 12% of tumors involved the whole corpus callosum. In detail, there were PCNL (66.6%), glioblastoma (16.7%), and germ cell tumor (16.7%) as shown in Figure 1e.
Hypervascularization of the tumor was noticed in glioma while there were no signs of flow void seen in lymphoma or germ cell tumors (Chi-square test, P < 0.001). Two-thirds (58.8%) of the hypervascular configurations were found in glioblastoma whereas the remaining configurations were diffuse astrocytoma (23.5%), anaplastic astrocytoma (11.8%), and gemistrocytic astrocytoma (5.9%). Almost half of tumor necrosis (44.8%) were observed in glioma and more than two-thirds (64.3%) had extensive necrosis (>50% of tumor size) in glioblastoma.
Table 2 demonstrates the treatment and outcome of butterfly tumors. Surgical management was the treatment of choice in butterfly tumors because histological diagnosis should be confirmed. The majority of operations were flameless navigator-guided biopsies; there were no cases of total tumor resection. Adjuvant therapy after surgery was radiation and chemotherapy. According to histology, several regimens of chemotherapy were chosen in 34% of cases. Unfortunately, the general outcome of butterfly tumor was quite poor. Half of the patients developed progressive diseases. The mortality rate was 78%.
Survival of butterfly tumor
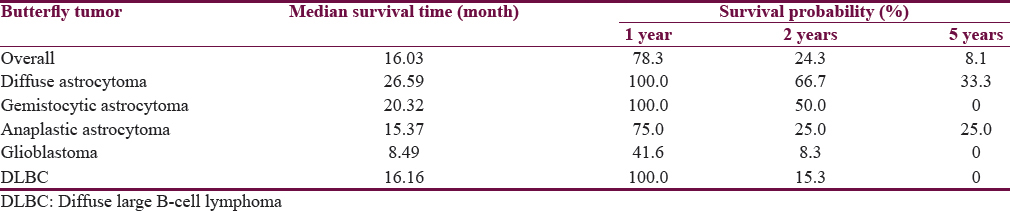
The prognosis of butterfly tumor was pitiable as shown in Figure 2a. Thus, the overall median survival time was 16.03 (95% confidence interval [CI]: 14.0–19.8), while the 1-, 2-, and 5-year survival probability were 78.3, 24.3, and 8.1, respectively. Allowing for histology, glioblastoma was the most fatal tumor with a very poor prognosis. The median survival time of the disease was 8.49 (95% CI: 1.47–17.40), whereas diffuse astrocytoma had a favorable prognosis. In addition, anaplastic astrocytoma had a survival similar with PCNL as shown in Table 3.
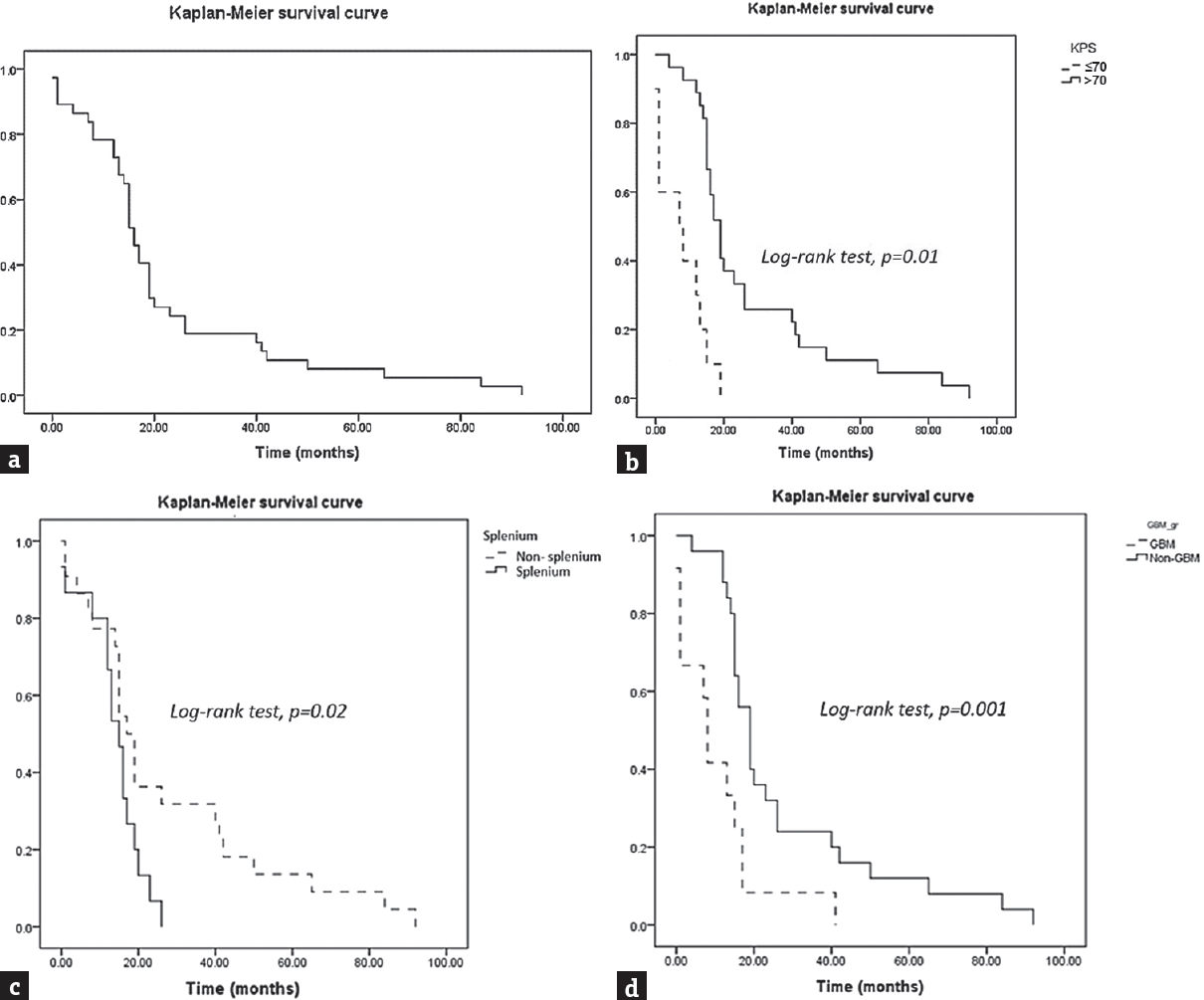
- Survival of the butterfly tumor according to prognostic factors using Kaplan–Meier curves and log-rank tests. (a) Overall survival curve with the median overall survival 16.03 months (95% confidence interval: 14.0–19.8). (b) The patients who had Karnofsky Performance Status >70 (solid line) and Karnofsky Performance Status ≤70 (dashed line). (c) Splenium cluster (solid line) and nonsplenium cluster (dashed line). (d) Glioblastoma subgroup (dashed line) and nonglioblastoma subgroup (solid line)
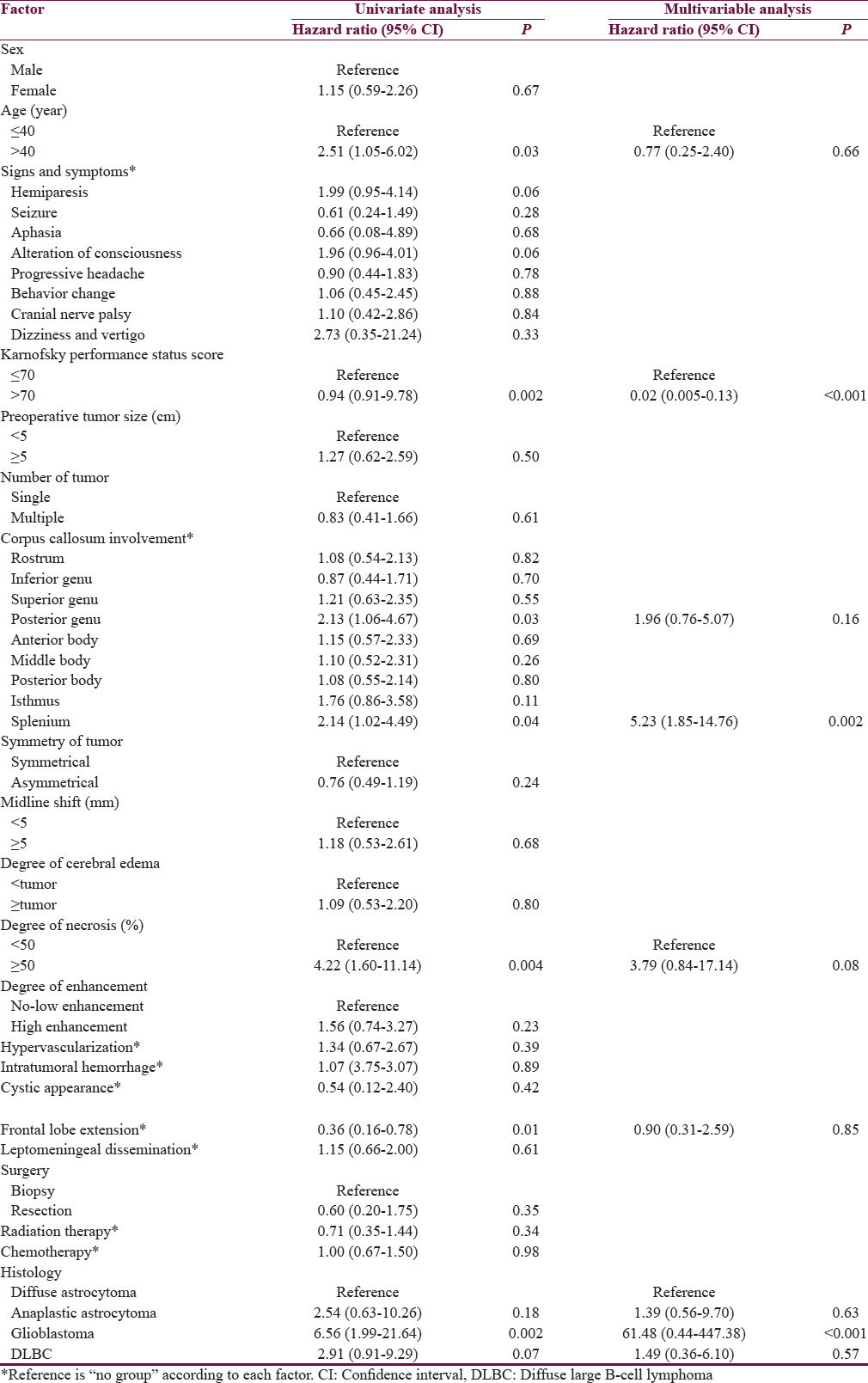
According to the aim of the study, the Cox proportional hazard regression model was used to analyze each factor which correlated with survival. Table 4 shows the results of both univariate and multivariate analysis. Initially, the significant factors were age >40 years, KPS score ≤70, degree of tumor necrosis, tumor at posterior genu and splenium, tumor extended to frontal lobe, and glioblastoma in univariate analysis. KPS score ≤70, splenium tumor, and glioblastoma were significant predictors associated with death. Finally, the Kaplan–Meier survival curves were constructed and log-rank tests were used to analyze the factors associated with death. Finally, KPS score ≤70, splenium involvement, and glioblastoma were significant predictors associated with death as shown in Figure 2b–d.
DISCUSSION
The intracranial neoplasms involving the bilateral corpus callosum construct a midline configuration in the axial plain which looks like a butterfly in the head. The differential diagnoses in these lesions are infection,[13] trauma (diffuse axonal injury),[14] demyelinating disease,[15] or tumor.[34] Therefore, the majority of butterfly lesions are primary malignant tumors, especially PCNL. In the literature, butterfly glioblastomas have frequently been mentioned.[31617] Conversely, diffuse large B-cell lymphomas were more common than glioblastomas in the present study.
Considering the clinical manifestations of these tumors, progressive headaches, and motor weakness are common presentations due to large tumors, perhaps, extending into the motor pathway. In spite of a common tumor invading at the genu, the splenium was a significant location for predicting prognosis. The corpus callosum has complex fibers connected to both the cerebral interhemispheres. Based on diffuse tensor imaging, Rimkus et al. studied the neuronal damage of the corpus callosum in relapsing-remitting multiple sclerosis. The destruction of the corpus callosum significantly increased in the posterior midbody and splenium.[18] Acceleration loading involved the corpus callosum, especially in genu, was correlated with unfavorable outcome in traumatic brain injuries.[141920] Contrariwise, the tumor invading the splenium of the corpus callosum is associated with poor outcome in multivariate analysis. As the splenium realized in several associative pathways is associated with memory and cognitive function, the pathology at this region made for worse prognosis.[21]
Furthermore, patient performance status is the key to favorable outcome and prognosis in neurooncology. Focusing primarily on B-cell central nervous system (CNS) lymphoma, poor performance status was a significant factor associated with prognosis. Moreover, lymphoma involved the deep structure of the brain, such as corpus callosum, basal ganglion, and the brainstem and was significantly associated with poor survival.[4] In addition, butterfly lymphoma had a median survival time shorter than nondeep-seated PCNL (16.16 months vs. 35–52 months,[48] respectively).
Considering the patients with glioma, previous studies reported the clinical characteristics of low-grade glioma associated with negative factors included being aged >40 years at diagnosis, presence of neurologic deficit before surgery seizure, diameter of the tumor >6 cm, KPS score <70, tumor crossing the midline, and astrocytoma histology subtype.[222324]
High-grade glioma has negative factors, including being aged >50 years, KPS score <70, glioblastoma subtype, tumor resection >98%, postoperative radiation, and temozolomide in the glioblastoma subtype.[12252627]
In multivariable analysis, glioblastoma histology subtype was the unfavorable predictor of survival outcome. Considering glioma, the histologic subtype and grading demonstrated the significant prognostic value.[28] The median overall survival was between 100 months and 10.5 years for the patient with low-grade glioma,[2223] 16 months for anaplastic astrocytoma,[25] and 6–14 months for glioblastoma.[2930]
We reported the median survival of butterfly glioblastoma was 8.49 months, which is similar to the previous study,[3] whereas patients with nonbutterfly glioblastoma who received a biopsy had a median survival between 6.3 and 6.6 months.[2931] Limitations of these clusters are unresectable. Lacroix et al. mentioned the extensive tumor resection of more than 98% that improved the prognosis in multivariable analysis.[12] Perhaps, surgical management was inadequate; thus, tumor load influenced more than adjuvant therapy. Therefore, the type of adjuvant chemotherapy was not a significant factor.
For glioma, the classification of the CNS tumor was revised in 2016. One of the new entities is the diffuse midline glioma which often involves the brainstem, spinal cord, or thalamus and is commonly found in the pediatric population. In addition, these tumors are associated with K27M mutations in the gene H3F3A.[323334] Although the butterfly gliomas are in the anatomical midline, these tumors are not mentioned in the new entity. Furthermore, the corpus callosum gliomas are mainly established in adults. The authors hypothesize that butterfly glioma might be a subcategory of diffuse glioma. Furthermore, IDH status has recently been used to predict prognosis in glioma. Zakrzewska et al. reported that the molecular profiles of butterfly glioblastoma were not associated with TP53, epidermal growth factor receptor, and MDM2 alterations. However, the right hemispheric tumor had a high level of microsatellite instability and loss of heterozygosity (LOH) in chromosome 5q, 9p, and 13q while the left side tumors had LOH in chromosome 3p, 5q, 9p, 9q, 10p, 10q, and 13q.[17] The molecular investigation in these types of tumors needs to be explored for a descriptive unique subtype.
The other limitations of the present study are its retrospective methodology and population size; however, this method is appropriate for the unusual manifestation of disease. Clarifying definitions before review helps diminish bias. On the contrary, the strengths of this present study include focusing on strong outcome. Mortality is solid evidence in which to assess outcome and prognosis.
CONCLUSIONS
This study provides a widespread assessment of butterfly lesions, including clinical characteristics, treatment, outcome, and prognosis. The results highlight the factors which significantly impact survival, including good performance status, splenium tumor involvement, and glioblastoma cluster. Furthermore, butterfly glioma might be organized into a specific cohort. Genetic profiling is feasibly useful to distinguish between diffuse midline glioma and butterfly glioma subtype in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol. 2002;179:251-7.
- [Google Scholar]
- Butterfly glioblastomas: A retrospective review and qualitative assessment of outcomes. J Neurooncol. 2012;109:555-63.
- [Google Scholar]
- Non-deep-seated primary CNS lymphoma: Therapeutic responses and a molecular signature. J Neurooncol. 2014;117:261-8.
- [Google Scholar]
- Butterfly lesion of the corpus callosum: An unusual case of extramedullary myeloid sarcoma (granulocytic sarcoma) Clin Nucl Med. 2011;36:365-6.
- [Google Scholar]
- Atypical imaging appearance of toxoplasmosis in an HIV patient as a butterfly lesion. J Magn Reson Imaging. 2009;30:873-5.
- [Google Scholar]
- Adult neuronal ceroid lipofuscinosis with clinical findings consistent with a butterfly glioma. Case report. J Neurosurg. 1998;88:314-8.
- [Google Scholar]
- Primary B-cell CNS lymphoma clinicopathologic and treatment outcomes in 89 patients from a single tertiary care center. Int J Hematol. 2014;99:450-6.
- [Google Scholar]
- The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: A post-mortem study. Brain. 1999;122(Pt 1):99-110.
- [Google Scholar]
- The use of the nitrogen mustards in the palliative treatment of carcinoma – With particular reference to bronchogenic carcinoma. Cancer. 1948;1:634-56.
- [Google Scholar]
- “Flow-void” sign at MR imaging: A rare finding of extracranial head and neck schwannomas. J Magn Reson Imaging. 2010;31:703-5.
- [Google Scholar]
- A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-8.
- [Google Scholar]
- Mild encephalopathy with reversible lesions in the splenium of corpus callosum and bilateral cerebral deep white matter in identical twins. Pediatr Rep. 2016;8:6615.
- [Google Scholar]
- Corpus callosum lesions after closed head injury in children: MRI, clinical features and outcome. Neuroradiology. 1992;34:384-8.
- [Google Scholar]
- Teaching neuroimages: Demyelinating disease mimicking butterfly high-grade glioma. Neurology. 2010;75:e4-5.
- [Google Scholar]
- Butterfly glioma of corpus callosum presenting as catatonia. World J Biol Psychiatry. 2007;8:54-5.
- [Google Scholar]
- Diverse molecular pattern in a bihemispheric glioblastoma (butterfly glioma) in a 16-year-old boy. Cancer Genet Cytogenet. 2007;177:125-30.
- [Google Scholar]
- Segmented corpus callosum diffusivity correlates with the Expanded Disability Status Scale score in the early stages of relapsing-remitting multiple sclerosis. Clinics (Sao Paulo). 2013;68:1115-20.
- [Google Scholar]
- Prognostic value of corpus callosum injuries in severe head trauma. Acta Neurochir (Wien). 2017;159:25-32.
- [Google Scholar]
- Genu of corpus callosum in diffuse axonal injury induces a worse 1-year outcome in patients with traumatic brain injury. Acta Neurochir (Wien). 2011;153:1687-93.
- [Google Scholar]
- Supratentorial low-grade glioma in adults: An analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294-301.
- [Google Scholar]
- Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: A retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-40.
- [Google Scholar]
- Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076-84.
- [Google Scholar]
- Five-year follow-up results for patients diagnosed with anaplastic astrocytoma and effectiveness of concomitant therapy with temozolomide for recurrent anaplastic astrocytoma. Asian J Neurosurg. 2012;7:181-90.
- [Google Scholar]
- Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-66.
- [Google Scholar]
- Prognosis and patterns of care in elderly patients with glioma. Cancer. 2009;115:5534-40.
- [Google Scholar]
- Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79-87.
- [Google Scholar]
- Survival analysis of 205 patients with glioblastoma multiforme: Clinical characteristics, treatment and prognosis in China. J Clin Neurosci. 2009;16:1595-8.
- [Google Scholar]
- Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239-44.
- [Google Scholar]
- The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803-20.
- [Google Scholar]
- Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425-37.
- [Google Scholar]
- A sensitive and specific histopathologic prognostic marker for H3F3A K27M mutant pediatric glioblastomas. Acta Neuropathol. 2014;128:743-53.
- [Google Scholar]






