Translate this page into:
Assessment of topiramate's efficacy and safety in epilepsy
Address for correspondence: Dr. Prem P. Gupta, Department of Pharmacology, All India Institute of Medical Sciences (AIIMS), Patna, Bihar, India. E-mail: drprempgupta@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To study the significance of topiramate (TPM) addition on seizure control in treatment of epilepsy.
Design:
A prospective open label add-on trial of TPM addition in patients with epilepsy was done. The events of baseline phase of 12 weeks followed by titration and maintenance phases were recorded. Assessment of the number of seizure and emergent adverse effects was done by a monthly visit for each case.
Main Outcome Measures:
Reduction of more than 50% mean seizure frequency or response ratio of 0.33 was taken as the criteria for responders.
Statistical Analysis:
Normal Z-test for significance of differences between two proportions and Chi-square test for presence of association was applied and mean age, median duration, sex ratio, percentage prevalence were depicted.
Results:
Significant responses to TPM in both partial as well as generalized seizures were observed (Z = 6.66, P < 0.001 and Z = 4.185, P < 0.01). The effect was more pronounced in patients with partial seizures. However, the overall response was highly significant (Z = 7.839, P < 0.001). The best response was noted at the dose of 200–300 mg/day (Z = 6.708, P < 0.001). More than 35% cases of partial and generalized seizures reported more than 75% reduction levels. The drug was well tolerated in more than 65% cases for side effects on psychosis, giddiness, and anorexia. Mild side effects were seen only in about less than 35% cases.
Conclusions:
TPM was found as a significantly effective add-on anticonvulsant with some limitation or mild side effects.
Keywords
Antiepileptic drug
seizures
topiramate
Introduction
Management and control of epilepsy is still a challenge. Recently, surgical remedial measures for lesions opened a new era in the management of these patients.[123] However, paucity of comprehensive epilepsy care centers having facilities of epilepsy surgery limit its scope for majority of such patients, particularly in poorly developed areas. Therefore a search of newer antiepileptic drugs (AEDs) to control epilepsy continues. Topiramate (TPM) is one such compound with a known therapeutic potential in these patients. It is a novel sulphamate substituted monosaccharide[4] with multiple mechanisms of action contributing to its efficacy in several seizure types. So the efficacy and safety of TPM as add-on therapy is being extensively investigated in various types of clinical trials at several places.
Electrophysiological and biochemical studies on neurons have identified several properties of TPM contributing to its anticonvulsant effect. These include reduction of the frequency of action potential generation following subjection of hippocampal neurons to a sustained depolarization that produced repetitive neuronal firing;[5] potentiation of some gamma-aminobutyrate (GABA)-activated receptors (benzodiazipine insensitive receptors).[6] TPM is rapidly absorbed from the gastrointestinal tract and its absorption is unaffected by food. In addition, TPM inhibits some isoenzymes of carbonic anhydrase, although this pharmacological effect is thought to be a minor component of its antiepileptic activity.[78] Double-blind placebo-controlled trials had shown that TPM was consistently effective as an adjunctive therapy in adults with partial onset seizures with or without secondary generalization.[910] The kinetics of other antiepileptic medication was generally not affected by concomitant TPM therapy. Addition of topiramate at doses of 100-800 mg/day produced no clinically significant changes in the plasma concentration of carbamazepine, valproate, phenobarbitone, or primidone.[11121314]
Materials and Methods
To assess the safety and efficacy of TPM in patients of partial and generalized tonic–clonic seizures had been carried out in the departments of pharmacology and neurology of B.R.D Medical College, Gorakhpur. The diagnosis of the epilepsy was established on the basis of clinical history and electroencephalographic examination (EEG). All the seizures were classified according to the classification of seizures laid down by International League Against Epilepsy (ILAE, 1981).[15] Each epilepsy was classified according to syndromic classification projected ILAE (ILAE, 1989).[16] A total of 106 consenting patients attending Neurology out-patient department (OPD) whose age was beyond 2 years formed the study subjects. Patients with a treatable cause of seizures (e.g. a metabolic disturbance, toxic exposure, or an active infection) or Pseudoseizures and those with progressive neurological disorders who have hepatic, renal, and cardiac diseases and/or breast feeding and pregnant were excluded from the study.
Study design
Study consisted of two phases. In the first phase, that is, Baseline phase: Which consisted of 12 weeks, during which patients continued to take their AEDs. Patients were observed for the frequency of seizures, duration, and type of seizures during this phase. The second phase, that is, Treatment Phase: Which can be divided in two phases (a) Titration phase (b) Maintenance phase.
Titration phase
The initial dose of TPM was 50 mg/day (1 mg/kg) with fortnightly increments of 50 mg until the maximum effective or maximum tolerated dose was reached. The total tolerated dose was not allowed to exceed 500 mg. It was administered in twice a day regimen.
Maintenance phase
The maximum effective/tolerated dose reached during titration phase was allowed to continue for at least 6 months. Concomitant AEDs were reduced if seizure control was complete and TPM as monotherapy was tried.
Data collection and analysis
At each study visit, the monthly seizure counts based on a review of patient diaries and treatment emergent adverse events, including intercurrent illness and injury reported by the patients and dosages of TPM and concomitant AEDs were recorded. EEG and biochemical tests for hepatorenal functions were done as and when required.
Efficacy assessment and statistical analysis
Patients were classified basically in two groups, as responders and nonresponders. The classification was based on ≥ 50% reduction in the baseline mean seizures frequency in a month or those with a response ratio of 0.33 or less. Response ratio is the difference between seizures frequency in the treatment (T) and baseline periods (B) divided by their sum (T-B/T + B). Negative values indicate fewer seizures.
The percentage difference among the responders and the nonresponders group as a whole and as per the type of seizures was evaluated for statistical significance by calculating Normal Z-test.
Results
A total of 106 patients were studied of which 70 were males and 36 were females. Of these, 40.56% patients were having epilepsy for less than 5 years and the maximum number 53.77% cases were having it from 5 to 20 years duration. Median duration of epilepsy was 7.6 years [Table 1].
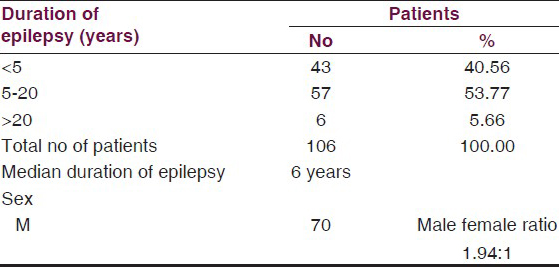
The mean age of the patients was 21.45 ± 10.24 years. Age-wise distribution is given in Table 2. A highly significant difference was observed between prevalence of cases 17.92% above and 82.07% below 20 years of age (Z = 9.345 P < 0.001).
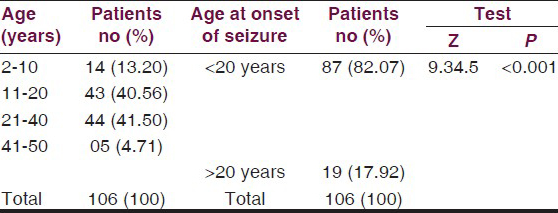
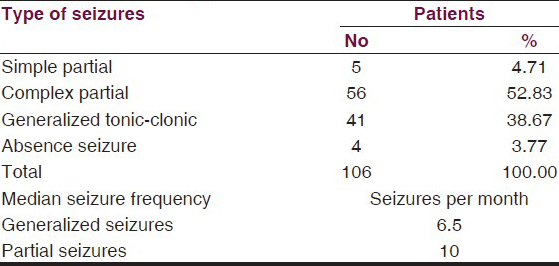
Complex partial seizures were the commonest 52.83%, while 38.7% had generalized tonic–clonic seizures, 4.71% had simple partial seizures and 3.77% had absence seizures. Median baseline seizure frequency per month for partial seizure was 10 seizures per month and 6.5 seizures per month for generalized tonic–clonic seizures. The baseline characteristics of patients are summarized in Table 3.
Of the total patients enrolled, 7.55% patients were on one concomitant AED 54.71% patients were on two AEDs, while 37.73% patients were on more than two AEDs. The computed tomography (CT) scan was done in 69.81% cases and EEG tests in all cases, but it showed insignificant differences between proportions of normal and abnormal findings [Table 4].
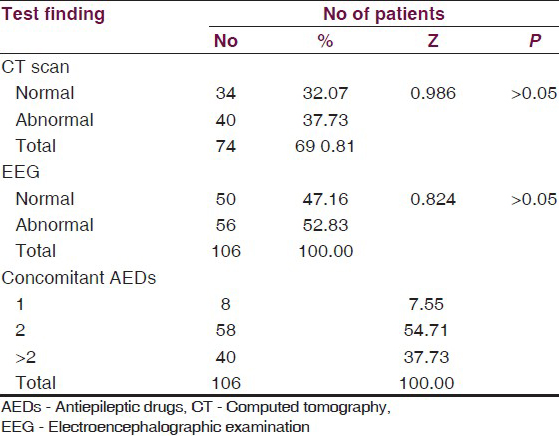
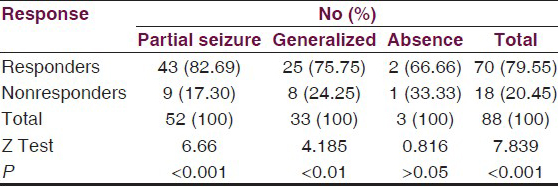
During the study period, 10 cases dropped out from the trial for different reasons, and in 8 cases, drug was withdrawn due to adverse effects, thus 88 (83.0%) patients completed the trial. Of all cases, 79.55% responded well to the treatment and 20.45% were nonresponders. The most highly significant response (82.69%) was from cases with partial seizures (Z = 6.65 P < 0.001) followed by significant response (75.75%) from generalized seizures (Z = 4.185, P < 0.05), but insignifant where seizures were absent [Table 5].
Efficacy
Out of the 106 patients, 88 patients entered the maintenance phase of these; 79.55% patients were found to be responders. The responder rate was 82.69% for partial seizures, 75.75% for generalized seizures, and 66.66% for absence seizures [Table 5]. The difference between responders and nonresponders was highly significant statistically in group as a whole and in patients with partial seizures and generalized seizures. The difference was most highly significant in patients with partial seizures, however, it was not statistically significant in patients with absence seizures.
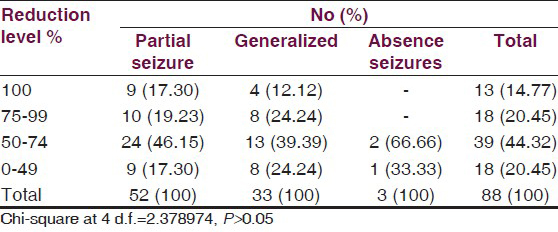
Percentage reduction in monthly seizure rate is summarized in Table 6. Among the 52 patients having partial seizures who entered the treatment phase responders were 82.69% [Table 5]; of these, 46.15% patients had shown reduction in seizure frequency between 50% and 75% and 19.23% patients showed 75-99% reduction, while 17.30% patients became totally seizure free. Out of 25 responders having generalized seizures [Table 5], 39.39% patients had shown 50-75% reduction in seizure frequency, 24.24% patients showed 75-99% reduction in seizure frequency, while 12.2% patients became totally seizure free.
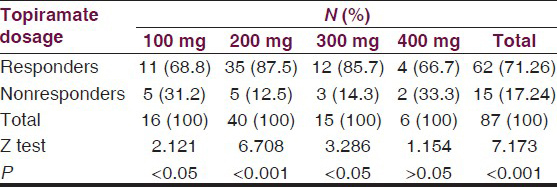
The minimum effective dose of TPM was 100 mg/day [Table 7] represent the response by maximal TPM dosages of 100, 200, 300, and 400 mg/day, respectively. Best response to TPM therapy in terms of percentage responders was noted with TPM dose of 200 mg/day. The difference in number of responders with a dose of 200 mg/day was most highly significant (Z = 6.708, P < 0.001) followed by significant responses of 100 and 300 mg/day but the dose of 400 mg/day was not significant statistically (Z = 1.154, P > 0.05).
Safety
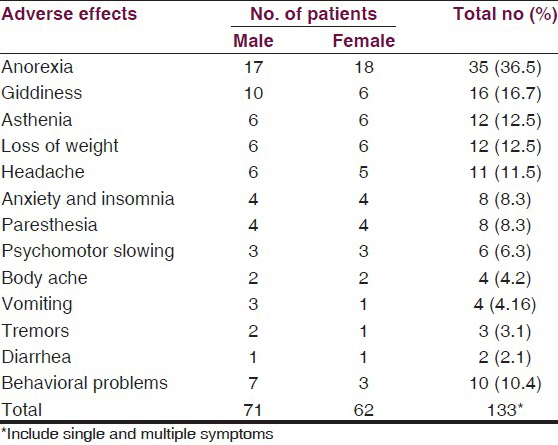
The most common adverse effects reported were central nervous system (CNS), related in 36.5% patients in the form of Anorexia. The next important adverse effects were giddiness, found in 16.7%, asthenia and loss of weight in 12.5% each, headache in 11.5% cases, anxiety and insomnia and paresthesia in 8.3% each, followed by infrequent psychomotor slowing, body ache, vomiting, tremors, diarrhea, which were found in only 2–6%. However, behavioral abnormalities were observed in at most 10.4% cases. The list of adverse effects is serially summarized in Table 8.
Discussion
For ethical reasons, the randomized controlled trials, which are the gold standard for demonstrating clinical ability of any drug, are difficult to conduct in patients with epilepsy, because it is not possible to maximize patient response fully. In such clinical trials of new AEDs, we can typically use a surrogate marker of 50% reduction in seizure frequency as primary end point. The present prospective open label study was intended to study the efficacy of TPM as add-on therapy and to recognize its adverse effects in patients with difficult to control seizures. Our results clearly show that TPM has significant effect in controlling seizures of all types. The effect being more pronounced in patients with partial seizures [Table 5]. The results have been similar in various open label and double-blind controlled trials.[1718192021] The therapeutic effect of TPM in our study was consistent regardless of age, gender, and baseline seizure rate.
Indian trial[22] endorse the efficacy of TPM in patients with partial seizures not controlled with other antiepileptics (such as phenytoin, carbamazepine, valproate, phenobarbitone, gabapentin, etc.). In our study, 88 out of 106 patients were either on one or more AED, but were still suffering from seizures. Out of these patients who entered the maintenance phase, 17.30% with partial seizures and 12.12% patients with generalized seizures became completely seizure free [Table 6] and as many as 82.69% patients with partial seizures, 75.75% with generalized and 66.6% patients with absence seizure responded positively [Table 5]. These findings have been corroborated by other studies as well[1920212223] [Table 1].
Reife et al.[23] in his pooled analysis of TPM did not record any patient characteristic that would have predicted a diminished or heightened response to TPM. The TPM was found to be equally effective in reducing the frequency of simple partial, complex partial, and secondarily generalized tonic–clonic seizures. The therapeutic effect was found to be consistent regardless of age, gender, and baseline seizure rate. No particular combination of TPM and another AED was found to be more efficacious or better tolerated. Our study endorses this experience as they contained patients of both sexes and the youngest patient was 5 years old. TPM has led to a substantial seizure reduction irrespective of baseline seizure frequency and type.
There are indications that TPM has the potential for use as a monotherapy in controlling seizures. Therefore an attempt can be made to discontinue concomitant AEDs therapy once the seizures are controlled. In reports from two study centers, 41 of the total 129 patients (32%) were converted to TPM monotherapy.[2425] These patients included 26 patients (63%) who were seizure free for at least 3 months and 7 patients who were experiencing simple partial seizure. We could achieve monotherapy in four of our patients who had simple partial seizures. Concomitant withdrawal of other AEDs was achieved in majority of our patients, that is, 1 drug in 41 patients (46.6%), 2 drugs in 18 patients (20.45%), and 3 drugs in 4 patients (4.54%). In our study, the dosage of TPM varied from 50 to 500 mg/day but the maximum response to TPM was seen at the dosage of 200 mg/day at which 87.5% and 300 mg/day at which 85.7% patients responded positively [Table 7]. The difference in responders with different dosages reached statistical significance too. A pooled analysis of TPM,[26] which has suggested 200 mg of TPM as a good target dose for add-on therapy in most patients with an excellent balance between efficacy and tolerability has been confirmed in our study too. However, randomized controlled trials[2728] have demonstrated that patients tolerability and retention on TPM therapy was improved when TPM was titrated with starting dosages of 50 mg/day without delaying the onset of the therapeutic effect. In our study, we have started the TPM dosage at 50 mg/day with fortnightly increments until the maximum effective or tolerated dosages were achieved.
Conclusion
To conclude, TPM has been shown as a generally effective and well tolerated add-on therapy in the control of seizures of all types, especially partial seizures. The optimum antiepileptic response is seen at a dose of 200 mg/day with an evident need to titrate the dose according to each patient. The study shows that the efficacy and safety profile of the drug in the population studied is similar to that seen elsewhere.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- General principles. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies (2nd ed). New York: Raven Press; 1993. p. :137-53.
- [Google Scholar]
- Foramen ovale and peg electrodes. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies (2nd ed). New York: Raven Press; 1993. p. :331-9.
- [Google Scholar]
- Selective effects of topiramate on sustained repetitive firing and spontaneous bursting in cultured hippocampal neurons. Epilepsia. 1993;34(Suppl 2):123.
- [Google Scholar]
- The novel anti-convulsant topiramate enhances GABA-mediated chloride flux. Epilepsia. 1993;34(Suppl 2):122-3.
- [Google Scholar]
- Double blind placebo-controlled trials of topiramate as add-on therapy in patients with refractory partial seizures. Epilepsia. 1996;37:539-43.
- [Google Scholar]
- Double-blind, placebo-controlled trial of topiramate (600 mg daily) for the treatment of refractory partial epilepsy. Epilepsia. 1996;37:763-8.
- [Google Scholar]
- Long-term follow up of patients treated with topiramate for partial seizures. Epilepsia. 1995;36(Suppl 3):S152.
- [Google Scholar]
- Pharmacokinetics and interaction profile of topiramate: Review and comparison with other new antiepileptic drugs. Epilepsia. 1997;38(Suppl 1):S18-23.
- [Google Scholar]
- Steady-state pharmacokinetics of topiramate and carbamazepine in patients with epilepsy during monotherapy and concomitant therapy. Epilepsia. 1996;37:774-80.
- [Google Scholar]
- The steady state (SS) pharmacokinetics (PK) of phenytoin (Dilantin) and topiramate (Topomax) in epileptic patients on monotherapy and during combination therapy. Epilepsia. 1994;35(Suppl 8):54.
- [Google Scholar]
- Explanation and prediction of drug interactions with topiramate using a CYP450 inhibition spectrum. Epilepsia. 1995;36(Suppl 4):47.
- [Google Scholar]
- Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League against Epilepsy. Epilpepsia. 1981;22:489-501.
- [Google Scholar]
- Proposal for revised classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the international league against epilepsy. Epilepsia. 1989;30:389-99.
- [Google Scholar]
- Percentage and paired alternatives. In: Statistics at Square One. Plymonth, U.K: British Medical Association Latimer Trend and Company Ltd; 1987. p. :28-32.
- [Google Scholar]
- Topiramate in medically intractable partial epilepsies: Double-blind placebo-controlled randomized parallel group trial. Korean Topiramate Study Group. Epilepsia. 1999;40:1767-74.
- [Google Scholar]
- Nonfocal generalized tonic-clonic seizures: Response during long-term topiramate treatment. Topiramate YTC/YTCE Study group. Epilepsia. 2000;41(Suppl 1):S77-81.
- [Google Scholar]
- Topiramate in the long-term management of refractory epilepsy. Topiramate YOL Study Group. Epilepsia. 2000;41(Suppl 1):S72-6.
- [Google Scholar]
- Low-dose topiramate in adults with treatment- resistant partial-onset seizures. Acta Neurol Scand. 2002;106:183-9.
- [Google Scholar]
- Topiramate: A new safe and effective antiepileptic. J Indian Med Assoc. 2001;99:449-50.
- [Google Scholar]
- Topiramate as add-on therapy: Pooled analysis of randomized controlled trials in adults. Epilepsia. 2000;41(Supp l):S66-71.
- [Google Scholar]
- A pooled analysis of adjunctive topiramate in refractory partial epilepsy. Acta Neurol Scand. 2003;108:9-15.
- [Google Scholar]
- The beneficial effect of slowing the initial titration rate of topiramate. Neurology. 1997;48(Suppl 2):A39.
- [Google Scholar]
- Onset of anticonvulsant effect of topiramate, a new antiepileptic drug. Neurology. 1997;48:A37.28.
- [Google Scholar]






