Translate this page into:
Anatomical variations in intracranial arteries: Does gender matter?
*Corresponding author: Himanshu Kaushal, Department of Neurology, Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, India. himanshukaushal1993@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaushik Y, Goyal G, Jain J, Kaushal H, Srivastava A. Anatomical variations in intracranial arteries: Does gender matter? J Neurosci Rural Pract. doi: 10.25259/JNRP_438_2024
Abstract
Objectives
Anatomical variations in cerebral arterial morphology are common and can have significant clinical implications. Digital subtraction angiography (DSA) serves as a crucial tool for visualizing these variations and guiding interventions. This retrospective study aimed to analyze intracranial arterial variations using DSA and to explore their demographic details and review their clinical significance.
Materials and Methods
A total of 394 patients who underwent DSA for various indications were included in the study. Demographic data, comorbidities, and clinical symptoms were collected and analyzed. DSA images were reviewed to assess arterial morphology, including normal variants and variations such as fenestrations, duplications, hypoplasia, and aplasia. The patients were divided into two groups (male and female) based on gender.
Results
The majority of patients were in the age groups of 51–60 years and >60 years, with a male preponderance (64.3%). Arterial variations were observed in 54.31%, with the posterior cerebral artery (PCA) being the most commonly affected followed by the anterior cerebral artery (ACA) and middle cerebral artery (MCA). Fetal PCA was observed in 21%, often bilateral. Hypoplastic and aplastic ACA segments were found in 11.93% and 5.1% of subjects, respectively. MCA trifurcation was seen in 10.4% of subjects. Anatomic variation in PCA was significantly higher in females, whereas ACA variations were more common in males.
Conclusion
Fetal PCA, hypoplasia or aplasia of proximal ACA (A1) were the most common anatomical variations. Gender-specific anatomical variations were also identified with females having more predisposition for PCA variations whereas ACA and vertebral artery variations were more frequent amongst males.
Keywords
Anatomical variations
Circle of Willis
Digital subtraction angiography
Intracranial arteries
INTRODUCTION
Anatomical variations in cerebral arteries, such as fenestrations, duplications, persistent primitive fetal arteries, and hypoplasia or aplasia of segments, are common in the general population due to the complexity of intracranial circulation embryology. However, the clinical significance of these variants varies widely. For instance, fenestrations and duplications may predispose arteries to aneurysm development.[1] Conversely, occlusion of the azygos or bihemispheric anterior cerebral artery (ACA) can lead to hemispheric ischemia. In addition, individuals with a fetal origin of the posterior cerebral artery (PCA) and concurrent carotid artery atherosclerosis are at increased risk of ischemic events in the PCA territory. While many variations have limited clinical impact, understanding them can assist in surgical planning and prevent complications during endovascular and surgical procedures. Digital subtraction angiography (DSA) is considered the gold standard for assessing angioarchitecture, understanding arterial blood supply dynamics, and guiding endovascular interventions. Although there have been various studies conducted on intracranial anatomical variations, only a handful of them are based on cerebral DSA. This study was conducted with aims (1) to identify various anatomical variations in intracranial arteries using DSA and, (2) to identify differences in the occurrence of anatomical variations in either of the genders.
MATERIALS AND METHODS
This study was a retrospective study conducted in the Department of Neurology from August 2022 to March 2024 after approval by the Institutional Ethics Committee with a waiver of consent due to the retrospective nature of the study and anonymous data collection. A total of 429 consecutive patients were included in the study who underwent cerebral DSA irrespective of indication by a neurointerventional specialist. Out of the original 429 patients, 35 were excluded for reasons including incomplete imaging, vasculature issues proximal to the Circle of Willis (CoW), or moderate to severe spasms of vessels. Patients with Moyamoya disease were excluded from the present study. Demographic information of the patients was analyzed. Comorbidities including diabetes mellitus, hypertension, atherosclerotic cardiovascular, cerebrovascular, and peripheral vascular disease were identified using the international classification of diseases-10 codes at the time of angiography. Smoking status was determined based on documented history preangiography. Regarding the DSA procedure, images were taken through a transfemoral approach using a 5 Fr catheter in a fully equipped DSA unit. Contrast was manually injected, and images were captured at 2 frames/s. Anteroposterior, lateral images, oblique, and other images were taken as needed based on pathology observed during angiography. Intracranial arteries were graded as normal, hypoplastic (vessel size <30% of the ipsilateral distal vessel) or aplastic (not seen on the DSA image).[2]
Statistical analysis was conducted using the Statistical Package for the Social Sciences software version 24. The chi-square test was used for comparison of findings in both male and female groups. P < 0.05 was taken as significant. The rest of the findings were depicted as descriptive statistics.
RESULTS
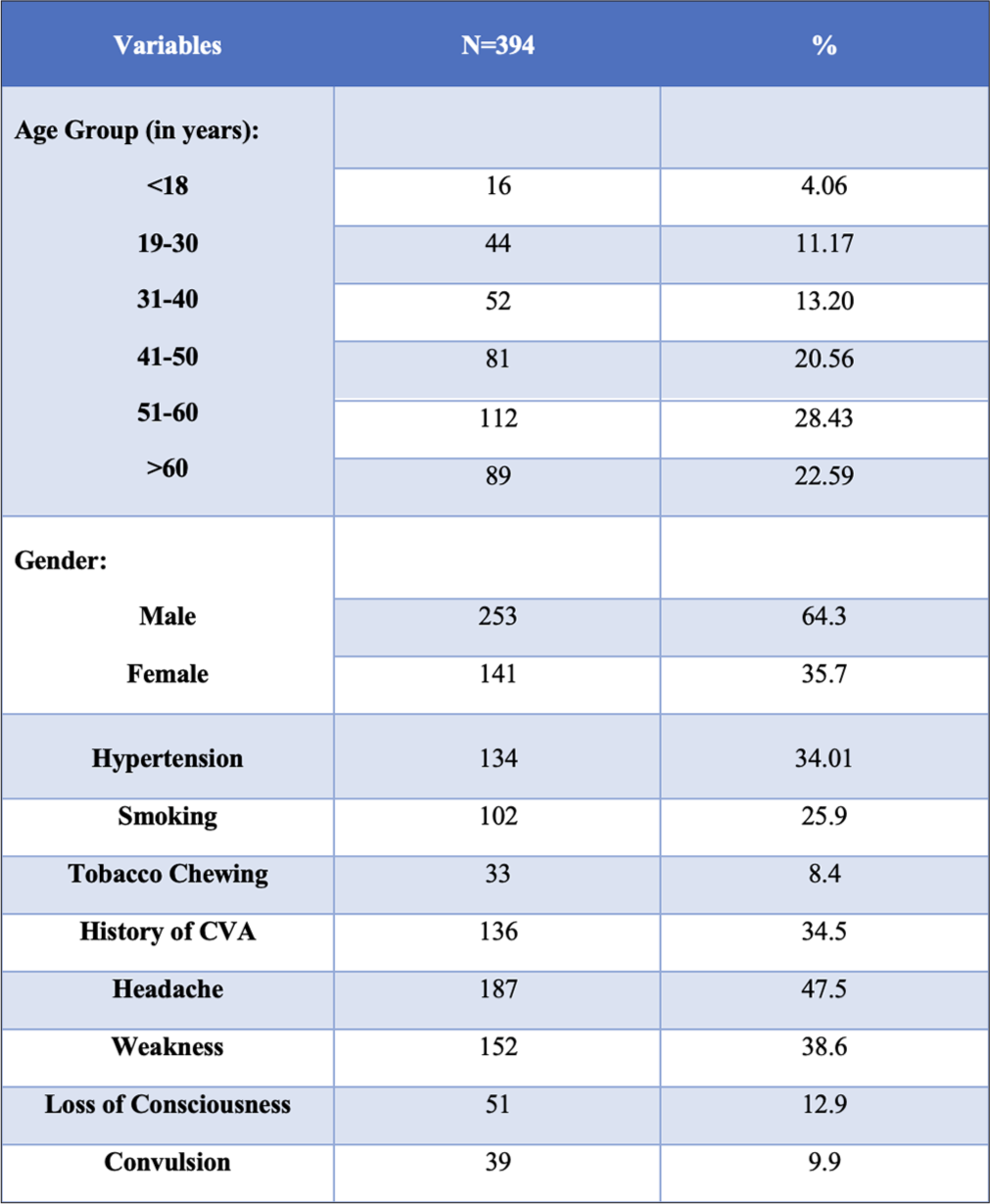
Original images of intracranial variations are shown in Figure 1. Among the total of 394 patients, the maximum were in the age group of 51–60 years (28.43%) and the minimum patients were from the age group of <18 years (4.06%). There was male preponderance in the study with males constituting 64.3% and females comprising 35.7%, respectively. About 34.01% of the patients were hypertensive. Headache, weakness, loss of consciousness, and convulsion, were reported in 47.5%, 38.6%, 12.9%, and 9.9% of patients, respectively [Figure 2].

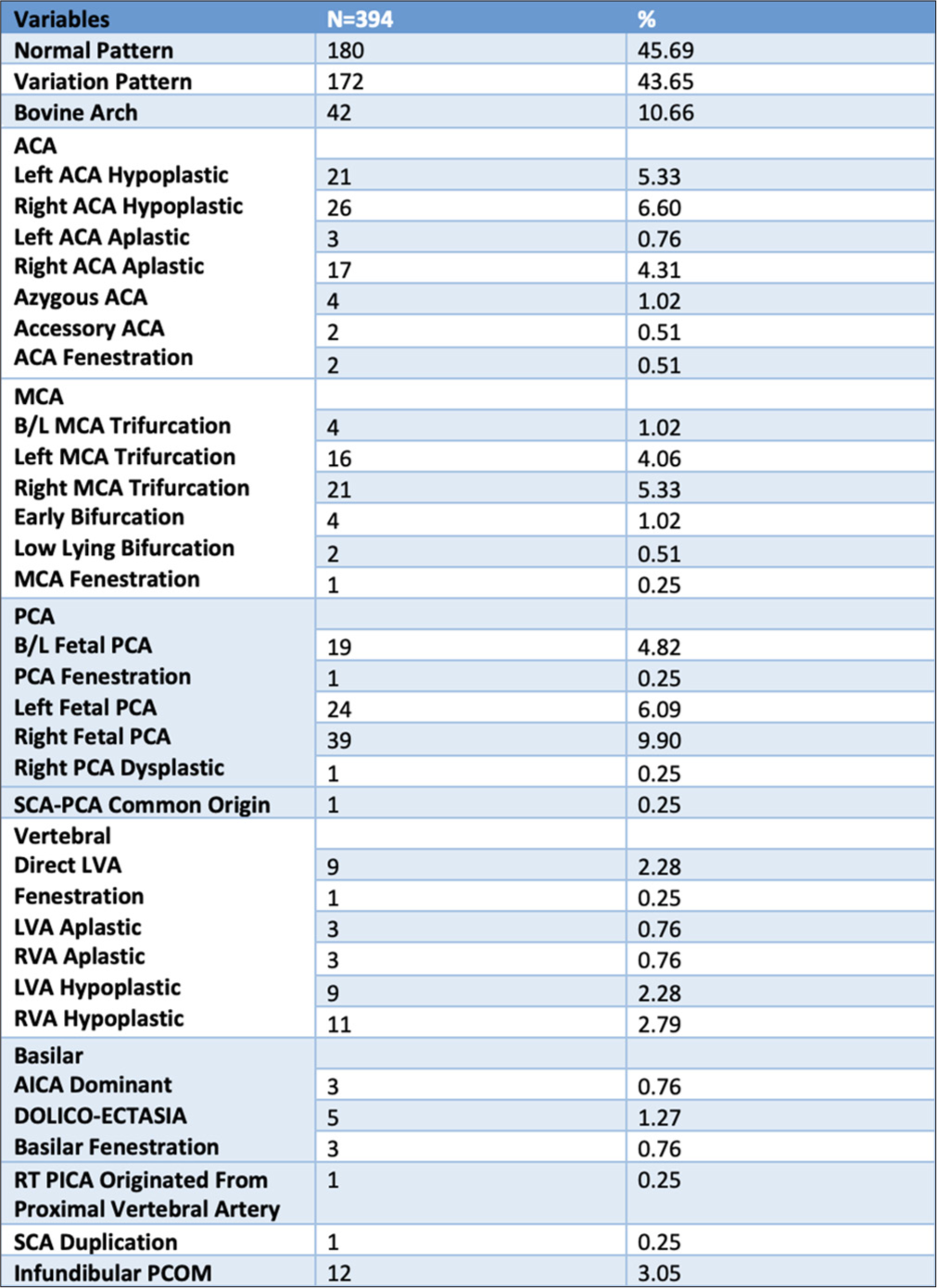
- Anatomical variations (shown by solid white arrows): (a) Aplastic anterior cerebral artery (ACA), (b) Azygous ACA, (c) Accessory ACA, (d) Hypoplastic ACA, (e) Dolichoectasiaof BA, (f) Dominant anterior inferior cerebellar artery, (g) Middle cerebral artery(MCA) trifurcation, (h) MCA fenestration, (i) BA fenestration, (j) Fetal origin of posteriorcerebral artery (PCA), and (k) Common origin of superior cerebellar artery-PCA.
- Branching pattern among the study patients. ACA: Anterior cerebral artery, MCA: Middle cerebral artery, PCA: Posterior cerebral artery, SCA: Superior cerebellar artery, B/L: Bilateral, LVA: Left vertebral artery, RVA: Right vertebral artery, AICA: Anterior inferior cerebellar artery, RT: Right, PICA: Posterior inferior cerebellar artery, PCOM: Posterior communicating artery.
Anatomical variation of cerebral arteries was found in 54.31% of total patients. Most commonly anatomical variation was observed in PCA, followed by ACA and middle cerebral artery (MCA). Fetal PCA was seen in 82 (21%) patients, 23% of these patients had bilateral fetal PCA. Hypoplastic and aplastic ACA was seen in 11.93% and 5.1% of patients, respectively. Azygos and accessory ACA were only seen in only four and two patients, respectively. MCA trifurcation was seen in 41 patients (10.4%), among these only four patients had bilateral MCA trifurcation. Fenestrations were observed in 7 patients (1.77%), most commonly seen in basilar artery (BA) (three patients). Dolichoectasia of BA was seen in five patients. Infundibular posterior communicating artery (PCOM) was reported among 3.05% of the patients. The bovine arch was seen in 10.66% of patients. Direct origin of the left vertebral artery (VA) was seen in 2.28% of patients. Hypoplastic and aplastic VA was seen in 5.1% and 1.5%, respectively. Detailed analysis of all variations is mentioned in Figure 3.
- Age, gender, and clinical profile among the study patients.
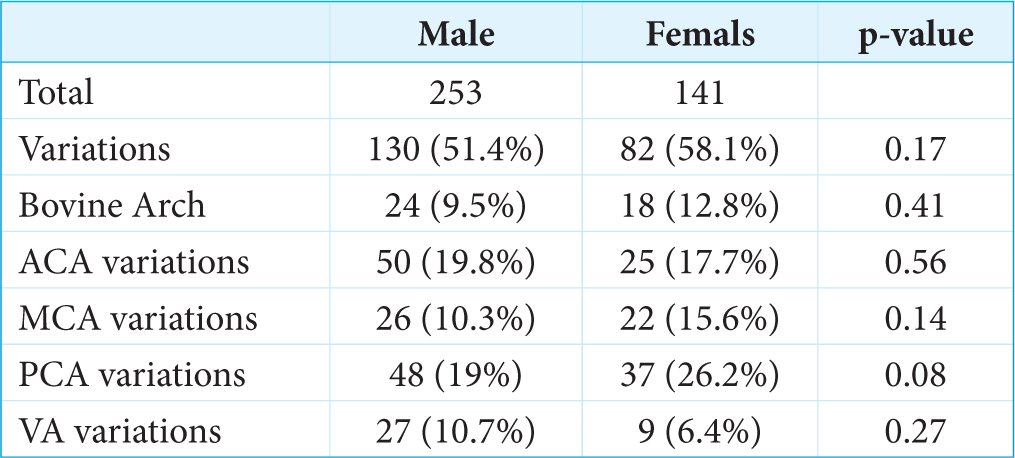
Among 141 females, 82 (58.1%) had anatomical variations whereas, in males, it was 51.4% (130 of 253). PCA variations were more common in females than males (26% vs. 19%), whereas variation in VA was more common in males than females (10.7% vs. 6.4%). Variations in specific gender are mentioned in Figure 4.
- Gender distribution of anatomical variations in study patients. ACA: Anterior cerebral artery, MCA: Middle cerebral artery, PCA: Posterior cerebral artery, VA: Vertebral artery.
DISCUSSION
Understanding the frequent occurrence of vascular pathologies in intracranial arteries underscores the importance of knowledge regarding their course and anatomical variations, particularly for intravascular and neurosurgical procedures.
Embryogenesis of vasculature of the brain is a complex process. It happens as a final outcome of various structural, functional, and metabolic processes. It is affected by the various areas of brain parenchyma and affected. Vasculogenesis and angiogenesis follow within 4–8 weeks after conception. The second branchial arch artery develops ventral and dorsal divisions, the latter of which joins the cranial dorsal aorta to produce the internal carotid artery (ICA). The external carotid artery is derived from the ventral part, which develops into the ventral pharyngeal artery. The hyoid artery, which temporarily produces the stapedial artery as a branch, is the dorsal branch that is attached to the ICA. The caroticotympanic artery is the remaining portion of the second branchial arch artery at the conclusion of development. The arteries of the third and fourth branchial arches emerge. The proximal ICAs are created when the third joins the distal paired dorsal aorta.[3]
The fetal origin of the PCA occurs when the embryonic PCA fails to regress. If a prominent PCOM is observed with the hypoplastic ipsilateral P1 segment of the PCA, it is classified as partial fetal origin; complete absence of the ipsilateral P1 segment is termed as complete fetal origin. The reported prevalence of fetal origin varies widely in cadaveric, DSA, and magnetic resonance angiographic studies, ranging from 11% to 29% for unilateral cases and 1% to 9% for bilateral cases. This variant may occur in approximately 10% of cases unilaterally and in 8% bilaterally. The posterior communicating arteries are crucial collaterals of the CoW, necessitating assessment of their patency before surgical and interventional procedures. Recognition of either type of fetal PCA variant is clinically significant due to its implications for cerebral blood supply, as the atheromatous disease of ICA in people with fetal PCA can cause stroke in PCA territory. In their study, Sahin and Pekçevik found that fetal-type PCA was the most common anatomical variation, present in 47.3% of patients. Bilateral absence of PCA was more frequent than unilateral absence, with unilateral absence predominantly occurring on the left side.[4] PCA fenestration is exceedingly rare, observed in only 0.25% of cases in our study.
Variations in ACA such as hypoplastic A1, aplastic A1, and azygos ACA can lead to reduced collateral supply during thromboembolic events. Azygos ACA is defined as a single unpaired A2 segment representing the persistence of the median artery of the corpus callosum. Azygos ACA anomaly is clinically significant and should be documented, as ACA occlusion from any cause can impact both hemispheres. In our study, hypoplastic and aplastic ACA were seen in 11.93% and 5.1% of patients, respectively, and azygos and accessory ACA were only seen in only four and two patients, respectively. Sahin and Pekçevik also reported that hypoplasia of the A1 segment was observed in 14.6% of cases, while its absence was noted in 2.53%.[4] In a cadaveric study of 112 preserved brains, 35% of the brains studied were found to have anatomical variations in ACA.[5]
Halama et al. identified that the most abundant ACA variants were those with aplastic or hypoplastic A1 segments, which formed 14.1% of the overall number (left: 6.2% and right: 7.5%). Hypoplastic or aplastic P1 segments (fetal type) were found in 12.4% of individuals with PCOM variations (left 5.3% and right 7.1%). The most frequent congenital carotidvertebrobasilar anastomosis, persistent trigeminal artery, was identified in two angiograms (0.4%). Early bifurcation of MCA was the most common variation in vascular branching, appearing in 42.8% of instances (left 23.2% and right 19.6%) of angiograms.[6]
Gender difference
In the present study, the overall difference in the occurrence of anatomical variations in male and female groups was found to be non-significant (P = 0.17). Although, among these anatomical variations bovine arch (9.5% males and 12.8% females; P = 0.41), ACA variations (19.8% males and 17.7% females; P = 0.56), MCA variations (10.3% males and 15.6% females; P = 0.14), PCA variations (19% males and 26.2% females; P = 0.08), and VA variations (10.7% males and 6.4% females; P = 0.27) were found with an insignificant statistical difference, as shown in Figure 3. Various neurological and psychiatric conditions have substantial differences in prevalence between males and females. In a meta-analysis that studied the gender differences in the morphology of the brain, males were found to have larger brain volume, intracranial volume, and gray and white matter.[7] There are limited data regarding gender differences in intracranial vasculature, especially from India. In a study of 411 patients, there was a higher incidence of ACA variations among females (23 vs. 16%).[8] Among the literature regarding the morphology of CoW, few studies reported a higher percentage of females having complete CoW,[9,10] whereas others reported no such correlation between female gender and CoW variation.[11,12] In a study of a cohort of South African patients, there was no statistically significant gender-specific difference in the morphology of the majority of the CoW arteries, except for the A1 segment of ACA.[13] In a meta-analysis of 14 studies regarding the anatomical difference of intracranial arteries, which included 5478 healthy participants (2511 women and 2967 men), variation in PCA was significantly higher in females with a relative risk of 2.79, whereas ACA variations were more common in males.[14] The findings in our study are consistent with this meta-analysis.
Limitation
The main limitation of the present study is that it is a single-centered study and the results of the same cannot be generalized to a population of wide diversity in the country. This study opens up further scope for future studies with similar aims to discover more complex and novel anatomic variations in the population.
CONCLUSION
The present study focused on identifying morphology and variations of intracranial arteries in Indian patients who had undergone cerebral DSA from a single center. A large number of study patients had different forms of variations. Fetal PCA, hypoplasia or aplasia of A1 were the most common anatomical variations. Gender-specific anatomical variations were also identified with females having more predisposition for PCA variations whereas ACA and VA variations were more frequent among males.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study, as all the data were gathered retrospectively and anonymously.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies. Am J Neuroradiol. 1993;14:675-80.
- [Google Scholar]
- Demographic age-related variation in circle of Willis completeness assessed by digital subtraction angiography. Brain Circ. 2020;6:31-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral neurovascular embryology, anatomic variations, and congenital brain arteriovenous lesions. J Neurointerv Surg. 2022;14:910-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical variations of the circle of Willis: Evaluation with CT angiography. Anatomy. 2018;12:20-6.
- [CrossRef] [Google Scholar]
- Variations of anterior cerebral artery in human cadavers. Neurol Asia. 2013;18:249.
- [Google Scholar]
- Reference values of cerebral artery diameters of the anterior circulation by digital subtraction angiography: A retrospective study. Diagnostics. 2022;12:2471.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34-50.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical variations of the anterior communicating artery complex: Gender relationship. Surg Radiol Anat. 2015;37:81-6.
- [CrossRef] [PubMed] [Google Scholar]
- Completion of the circle of Willis varies by gender, age, and indication for computed tomography angiography. World Neurosurg. 2017;106:953-63.
- [CrossRef] [PubMed] [Google Scholar]
- Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. 1998;207:103-111.
- [CrossRef] [PubMed] [Google Scholar]
- Variations in the circle of Willis in a large population sample using 3D TOF angiography. The Tromso study. PLoS One. 2020;15:e0241373.
- [CrossRef] [PubMed] [Google Scholar]
- A simple technique for morphological measurement of cerebral arterial circle variations using public domain software (Osiris) Anat Cell Biol. 2011;44:324-30.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring the anatomical configurations of the cerebral arteries in a cohort of South African patients. Sci Rep. 2024;14:6060.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical differences of intracranial arteries according to sex: A systematic review and meta-analysis. J Neuroradiol. 2024;51:10-5.
- [CrossRef] [PubMed] [Google Scholar]






