Translate this page into:
Anatomical considerations of cutaneous nerves of scalp for an effective anesthetic blockade for procedures on the scalp
*Corresponding author: Sipra Rout, Associate Professor, Department of Anatomy, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. siprarout@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Simon KS, Rout S, Lionel KR, Joel JJ, Daniel P. Anatomical considerations of cutaneous nerves of scalp for an effective anesthetic blockade for procedures on the scalp. J Neurosci Rural Pract 2023;14:62-9.
Abstract
Objective:
The anatomy of the scalp nerves varies widely with age, race, and individuals of the same race and even within the same individual and hence need to be studied extensively to avoid complications and improve effectiveness during various surgical and anesthetic procedures of the scalp.
Materials and Methods:
Gross dissection was carried out on 11 cadavers (22 Hemifaces: 11 right and 11 left) with no obvious scalp deformities or surgeries. The distances of the supraorbital nerve (SON), supratrochlear nerve (STN), and greater occipital nerve (GON) from commonly used bony landmarks were measured. The branching pattern and presence of accessory notches/foramina were noted.
Results:
SON and STN were found almost midway and at the junction between medial and middle one-third of the line joining midline and lateral orbital margin, respectively. The distances of STN and SON from the midline were about ½ and 3/4th of the transverse orbital diameters of the individual. GON was found at the medial 2/5 and lateral 3/5 of the line joining inion to the mastoid. In 40.9% cases, SON gave three branches while STN and GON remained as single trunks in 77.27% and 40.0% cases, respectively. Accessory foramina/notches for SON and STN were found in 36.36% and 4.54% of the specimen, respectively. SON and STN remained lateral in the majority while GON ran medially to corresponding vessels.
Conclusion:
These parameters on the Indian population would give a comprehensive idea of the distribution of these cutaneous scalp nerves and would be beneficial in the targeted and accurate deposition of local anesthetic.
Keywords
Supraorbital nerve
Cutaneous nerve
Greater occipital nerve
Scalp nerve block
INTRODUCTION
The use of local anesthetics to block the nerves supplying the scalp, often referred to as the “scalp block,” has experienced a renaissance over the past decade with the increasing complexity of neurosurgical procedures and interventions. The scalp block, frequently used as an adjunct to general anesthesia during neurosurgery, maintains stable intraoperative hemodynamics, reduces intraoperative opioid requirement facilitating early post-operative neurologic assessment, and provides perioperative analgesia.[1-3] Furthermore, with awake craniotomies being performed for resection of brain tumors residing close to eloquent areas of the brain such as the speech area or motor cortex, the scalp block is a “must-know” skill to be mastered by the anesthesiologist. Such tumor resections done with the scalp block being the sole anesthetic have shown better outcomes with regard to the extent of tumor resected, neurological outcomes, and duration of hospital stay.[4,5] Apart from the neurosurgical applications, the scalp block is vital in providing analgesia for extracranial procedures on the scalp.[6,7] Neuralgias, neuropathies, and headaches such as cluster, cervicogenic, and migraines are diagnosed and treated by blocking concerned nerves, which are components of the routine scalp block.[8]
The technique of the scalp block has evolved from being a field block, achieved by an indiscriminate ring blockade of the scalp, to a more focused and anatomically directed method, involving local anesthetic injections targeted to the specific nerves that innervate the scalp. This method, first demonstrated by Girvin, provides satisfactory analgesia and avoids the administration of an unnecessarily high volume of concentrated local anesthetic, reducing the risk of toxicity caused by systemic absorption.[9] Besides, it also avoids other complications such as nerve injury, intravascular injection of the anesthetic drug, brainstem apnea, hematoma formation at the injection site, and temporary facial nerve paralysis.[10-13] This highlights that a targeted approach to block the scalp nerves requires a thorough knowledge of the scalp nerve anatomy and its related structures. The cutaneous nerves that are blocked bilaterally are the four anterior nerves, namely, supratrochlear nerve (STN), supraorbital nerve (SON), zygomaticotemporal and auriculotemporal, and the three posterior nerves, posterior auricular, lesser, and greater occipital nerve (GON). Out of these, the SON and STN are commonly blocked for the procedures involving anterior region of the scalp and GON for the posterior region of the scalp as they supply the majority of the scalp region.[14]
Anatomical variations of peripheral nerves among populations have been well described.[15] The anatomy of scalp nerves varies widely with age (pediatric vs. adult population) among individuals of the same race and within the same individual bilaterally[16-28] and has not been studied extensively. These variations are also implicated in chronic headaches.[21,27-29] A thorough knowledge of the scalp nerve anatomy is paramount to maximize the benefits and prevent complications of the scalp block.
In this cadaver study, carried out in the Indian population, we aim at describing the topographical relation of the STN, SON, and the GON to their nearest bony landmarks, their branching patterns, and their relation to the corresponding vessels to provide congruous landmarks for scalp nerve blocks which would result in better anesthesia practice. To the best of our knowledge, there has been no prior similar dissection studies carried out on the Indian population. Thus, we feel, it will provide an enhanced understanding of the variations to be kept in mind while dealing with these nerves during procedures involving the scalp, thereby resulting in an effective block while also reducing complications.
MATERIALS AND METHODS
After approval from the Institutional Review Board, this study was conducted in the Department of Anatomy, Christian Medical College, Vellore, Tamil Nadu. The samples included 11 adult cadavers without any obvious scalp deformities or surgeries performed in this area. The STN, SON, and GON were dissected on both sides (22 hemifaces: 11 right, 11 left). Skin flaps were raised laterally from the midline to expose the STN and SON along with their branches. The nerves were traced back to their emergence from respective notches/foramen. In addition, accessory notches/foramina with emerging structures (if any) were noted. The relation of the nerves to their corresponding vessels and the number of branches given off by STN and SON within 1 cm of their emergence were observed. Posteriorly, skin flaps were raised from the midline and extended laterally till the emergence of the GON piercing the trapezius muscle was located. The nerve was then dissected up to the superior nuchal line. The branching pattern of GON and its relationship to the occipital artery was noted on the superior nuchal line. All the measurements were taken using a calibrated digital Vernier caliper with an accuracy of 0.001 mm. The results obtained were analyzed for their mean, standard deviation, ratios, maximum, and minimum values using standard formulae in Microsoft Office Excel 2007. Student’s t-test and paired t-test were used for calculating the p values for comparing the measurements on both the hemiscalps.
The schematic representation of the distances measured is shown in [Figure 1a and b].

- Schematic representation of the distances measured in the study.
RESULTS
Of the 11 cadavers, we dissected, five were males and six were females, ranging between 29 and 89 years. The mean age was 71.83 ± 22.24 years. The mean distances of midline (M) to the bony lateral orbital margin (LOM), Midline-medial orbital margin (M-MOM) and M to the bony MOM, distance between medial and LOM, and distance between inion (I) to the mastoid process (Ma) for this population are given in [Table 1]. There was no significant difference observed in comparing the distances between the two hemi-scalps.
| Distance (mm) | Overall | Right side (n=11) | Left side (n=11) | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | ||
| Midline-lateral orbital margin | 53.14±3.04 | 46.82-59.11 | 53.26±3.20 | 46.82-58.94 | 53.03±3.02 | 47.51-59.11 | 0.544 |
| Midline-medial orbital margin | 12.24±2.71 | 7.25-17.23 | 12.47±2.89 | 7.72-17.23 | 12.01±2.63 | 7.27-15.53 | 0.136 |
| Medial orbital margin-lateral orbital margin | 36.52±3.34 | 29.48-45.47 | 36.29±3.52 | 32.43-45.47 | 36.75±3.30 | 29.48-42.25 | 0.499 |
| Inion-Mastoid process | 86.04±5.81 | 73.62-94.79 | 85.78±5.86 | 73.88-92.64 | 86.29±6.03 | 73.62-94.79 | 0.149 |
M: Midline, LOM: Lateral orbital margin, MOM: Medial orbital margin, Ma: Mastoid process, I: Inion
The mean horizontal distance of STN and SON from midline was found to be 16.60 ± 2.39 mm and 25.51 ± 3.25 mm. We found a significant difference observed between both sides of STN and SON emergence point from the midline (M-STN) (P = 0.029) and M-SON (P = 0.022). The mean vertical distances on the orbital margin at the level of nasion (N) for STN and SON were found to be 4.32 ± 2.25 mm and 9.82 ± 2.62 mm, respectively. The proximity of SON to STN was found to be 8.91 ± 2.27 mm laterally and 7.09 ± 2.44 mm vertically. On the posterior aspect, the mean distances inion (I) to the mastoid process (Ma) and inion to I-GON were 86.04 ± 5.81mm and 34.92 ± 8.48 mm, respectively. There was no significant difference observed for the above measurements between both sides [Table 2].
| Distance (mm) | Overall | Rt (n=11) | Lt (n=11) | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | ||
| Midline-STN | 16.60±2.39 | 12.69–22.73 | 17.41±2.82 | 12.87–22.73 | 15.78±1.59 | 12.69–18.36 | 0.029 |
| Midline-SON | 25.51±3.25 | 21.11–32.31 | 26.72±3.77 | 21.11–32.31 | 24.29±2.18 | 21.82–28.63 | 0.022 |
| Nasion-STN | 4.32±2.25 | 0–8.45 | 4.18±2.11 | 0–7.58 | 4.45±2.49 | 0–8.45 | 0.411 |
| Nasion-SON | 9.82±2.62 | 4.2–13.91 | 9.70±2.49 | 4.41–13.91 | 9.94±2.87 | 4.2–13.89 | 0.769 |
| Horizontal distance (SON-STN) | 8.91±2.27 | 5.27–12.42 | 9.31±2.65 | 5.27–12.42 | 8.52±1.85 | 6.57–11.77 | 0.393 |
| Vertical distance (SON-STN) | 7.09±2.44 | 2.82–13.16 | 7.25±2.34 | 3.53–12.21 | 6.93±2.65 | 2.82–13.16 | 0.558 |
| Inion-GON | 34.92±8.48 | 21.73–50.82 | 34.85±8.95 | 21.73–49.95 | 34.99±8.42 | 21.76–50.82 | 0.929 |
| GON-Mastoid process | 51.12±9.23 | 32.8–64.76 | 50.94±9.31 | 37.62–63.34 | 51.30±9.61 | 32.8–64.76 | 0.830 |
STN: Supratrochlear nerve, N: Nasion, H (SON-STN)-horizontal distance between SON and STN, V (SON-STN)-vertical distance between SON and STN. The distance of the STN and SON from the mid-line on either side was found to be significant.
In most cases, SO and ST foramina were seen in variable combinations on either side for passage of respective neurovascular bundles [Figure 2a-g]. Accessory supraorbital and supratrochlear foramina were observed in 8 (36.36%) and 1 (4.54%) case, respectively [Table 3]. However, no significant structures involving accessory nerves were seen passing through these accessory foramina.
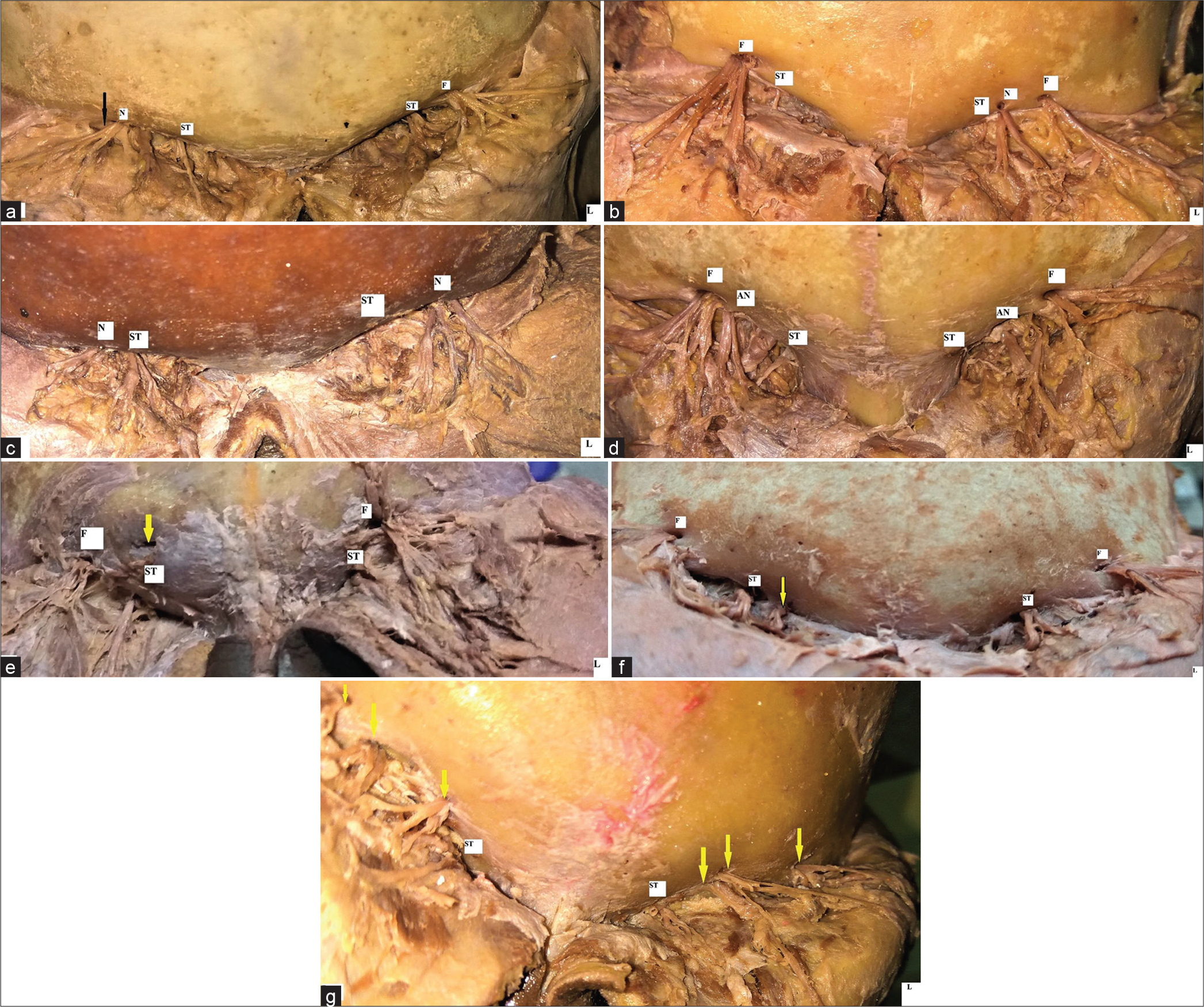
- Photographs showing variable occurrences of foramen and notches for SON and STN (a) Supraorbital notch (N) with a fibrous band (black arrow) on Right side. A well-defined supra orbital foramen (F) on left side (b) Presence of Supra orbital notch(N) on left side with Supra orbital foramen(F) on right side. ST: supratrochlear notch, (c) Supraorbital notch on(N) both the sides. ST: Supratrochlear notch, (d) Presence of accessory supraorbital notch(AN) and foramen(F) on both sides. One of the branches of SON was seen passing through the notch while other branches with vessel emerged through the foramen. ST: Supratrochlear notch, (e). Presence of an accessory foramen (arrow) without any significant structure passing through it. F: supraorbital foramen, ST: supratrochlear notch, (f) Presence of accessory supra trochlear notch (arrow) giving passage to one of branches of STN. F: supraorbital foramen, ST: supratrochlear notch, (g) Presence of multiple accessory foramens on both the sides showing emergence of branches of Supraorbital nerves. ST: supratrochlear notch.
| Accessory foramen and notches | Rt | Lt | Total | Incidence |
|---|---|---|---|---|
| SO | 4 | 4 | 8 | 36.36% |
| ST | 1 | Nil | 1 | 4.54% |
SO: Supraorbital, ST: Supratrochlear
At the level of the supraorbital ridge, SON (54.54%) and the STN (36.36%) were lateral in relation to corresponding vessels [Figure 3a]. However, posteriorly, GON was found related medially to the corresponding vessel at its emergence in 63.63% of cases [Figure 3b]. In the remaining, 13.63% of the vessels pass superficial to the GON [Figure 3c].
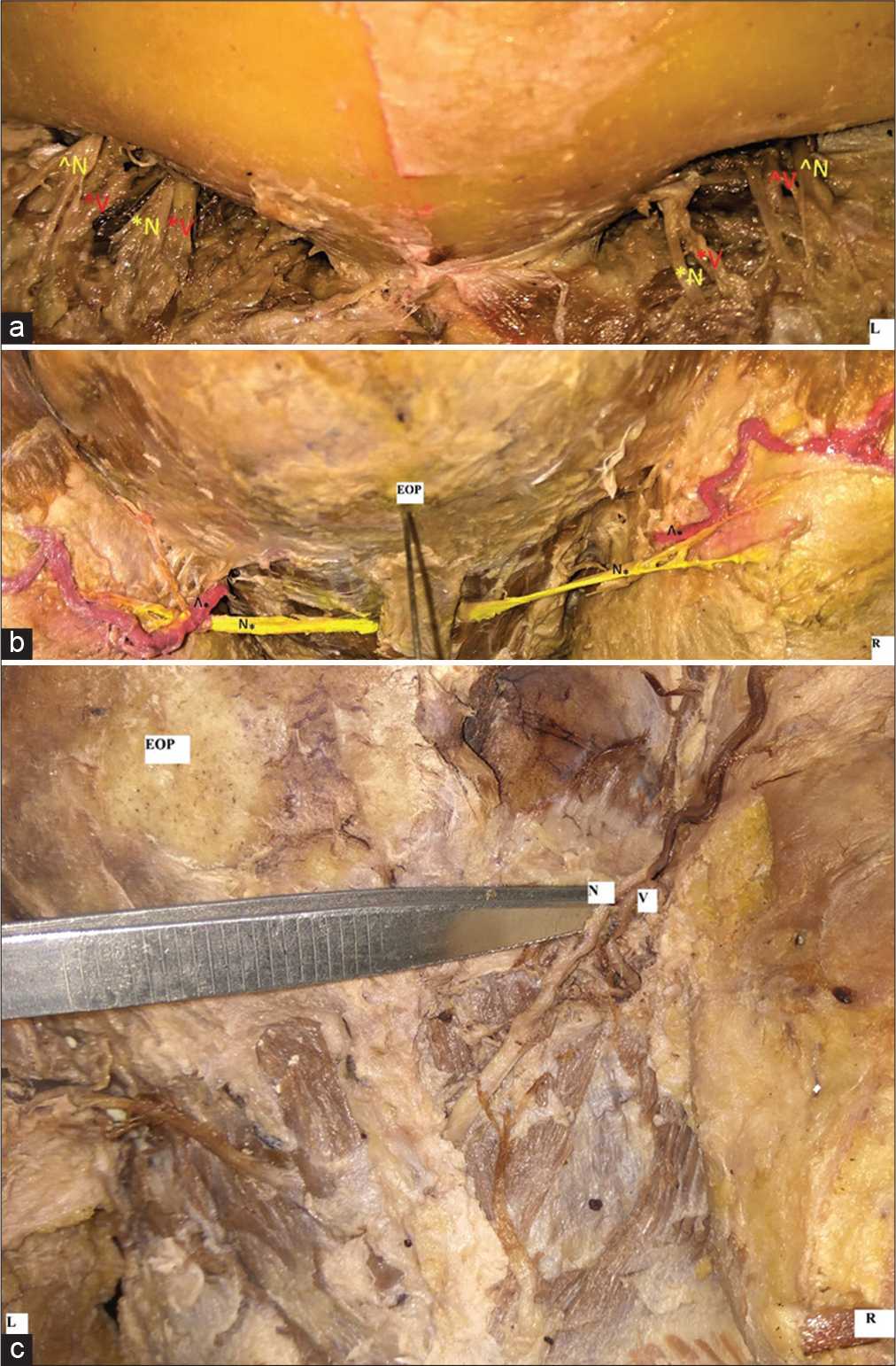
- (a-c) Photographs showing the relationship of SO, ST, GO to corresponding vessels at their emergence (a). Supraorbital nerve(N) lateral to the corresponding vessel on both sides. Supra trochlear nerve(N) is medial to its vessels on the left side while on right its lateral in relation (b). GON(N) medial and lateral to occipital artery (v)on right and left side respectively, (c) GON(N) passing superficial to occipital artery(v).
The number of branches given off by each nerve at its emergence varied greatly. The number of branches given for each nerve within 1 cm of its emergence is presented in [Table 4]. The GON also showed variation in its splitting pattern [Figure 4a-b and Table 5].
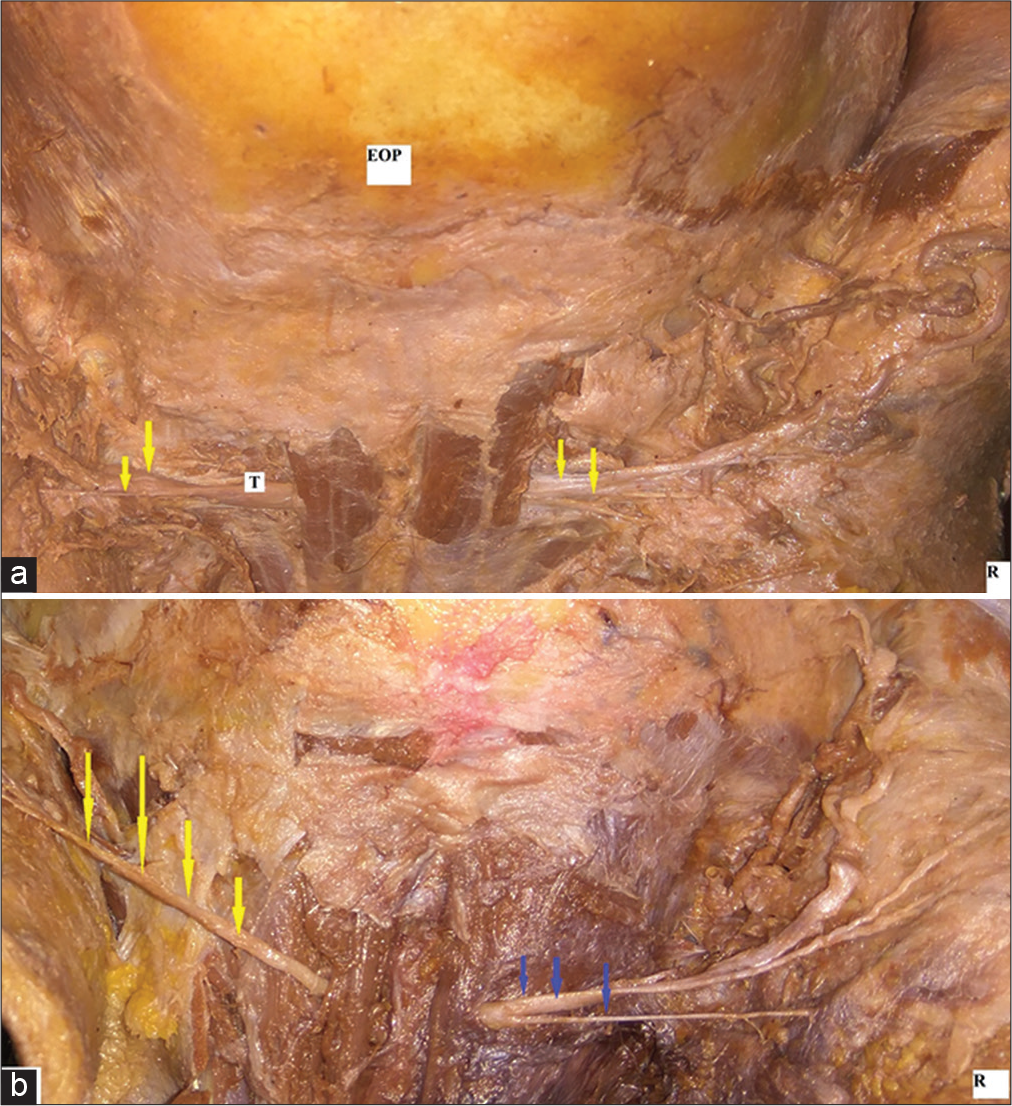
- (a-b) Photographs showing variations in splitting of GON seen (a) GON splitting after emerging from muscle (left side). GON seen emerging after splitting within the muscle (right side). T: trunk of greater occipital nerve (b) GON as a single unsplit trunk (left side). Split into multiple branches on right side.
| Nerves | Number of branches | ||||
|---|---|---|---|---|---|
| None | Single | Double | Triple | More than 3 | |
| STN | - | - | 36.36% | 40.9% | 22.72% |
| SON | 77.27% | 9.09% | 3.63% | - | - |
| GON | 40.9% | 22.72% | 13.63% | 18.18% | 4.55% |
STN: Supratrochlear Nerve, SON: Supraorbital Nerve, GON: Greater occipital Nerve
| Variation | Total (n=22) | Right (%) (n=11) | Left (%) (n=11) |
|---|---|---|---|
| Split before the emergence | 4 (18.18%) | 2 (18.18%) | 2 (18.18%) |
| Split at emergence | 1 (4.54%) | 1 (9.09%) | Nil |
| Unsplit at emergence | 17 (77.27%) | 8 (72.72%) | 9 (81.81%) |
The overall ratios between M-SON to M-LOM, M-STN to M-LOM, M-SON to MOM-LOM, and M-ST to MOMLOM were 0.48 ± 0.06 (range: 0.38–0.63), 0.31 ± 0.05 (range: 0.22–0.43), 0.70 ± 0.11 (range: 0.51–0.91), and 0.46 ± 0.09 (range: 0.28–0.69), respectively. These ratios compared between the right and left sides were statistically significant. However, the overall ratio of I-GON to I-Ma was 0.41 ± 0.10 (range: 0.25–0.55), and the comparison bilaterally is not significant statistically [Table 6]. There were no significant variations in the mentioned parameters between male and female cadavers.
| Distance (mm) | Overall | Right (n=11) | Left (n=11) | P-value | |||
|---|---|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | ||
| Midline -SON: Midline-LOM | 0.48±0.06 | 0.38–0.63 | 0.50±0.07 | 0.39–0.63 | 0.46±0.05 | 0.38–0.55 | 0.038 |
| Midline-ST: Midline-Lateral Orbital Margin | 0.31±0.05 | 0.22–0.43 | 0.33±0.06 | 0.22–0.43 | 0.30±0.03 | 0.24–0.34 | 0.043 |
| Midline-SON: Medial Orbital Margin-Lateral Orbital Margin | 0.70±0.11 | 0.51–0.91 | 0.74±0.12 | 0.51–0.91 | 0.67±0.08 | 0.53–0.83 | 0.010 |
| Midline-STN: Medial Orbital Margin-Lateral Orbital Margin | 0.46±0.09 | 0.28–0.69 | 0.49±0.10 | 0.28–0.69 | 0.43±0.07 | 0.32–0.60 | 0.012 |
| Inion-GON: Inion-Mastoid process | 0.41±0.10 | 0.25–0.55 | 0.41±0.10 | 0.25–0.54 | 0.41±0.1 | 0.25–0.55 | 0.987 |
M: Midline, LOM: Lateral orbital margin, MOM: Medial orbital margin, SON: Supraorbital nerve, STN: Supratrochlear nerve, GON: Greater occipital nerve
DISCUSSION
The SON, STN, and GON provide significant sensory supply to the scalp and are targeted and blocked more extensively than the other nerves in this region. The SON exits the cranium through the supraorbital foramen, ascends the forehead and provides sensory supply to the frontoparietal scalp[30] and up to the region of the lambdoidal suture while the STN supplies the scalp close to the midline.[31] The GON, the thickest cutaneous nerve in the body,[32,33] arises from the medial branch of the dorsal ramus of C2 and emerges below the obliquus capitis inferior muscle.[32] It variably pierces the inferior oblique, semispinalis, and trapezius muscles and provides sensory supply to the scalp over the occipital bone up to the vertex, the skin over the ear, and the area just above the parotid gland.[34-37]
In this study, we attempted to obtain a technique to locate the supraorbital, supratrochlear, and GONs, individualized to each patient based on their skull morphology in terms of bony landmarks and the size of the orbit. For this, the distances between the standard bony landmarks to the nerves under study are measured, and a correlation in the form of ratios was derived to observe if the distribution of the nerves follows a particular pattern with respect to the bony landmarks. At present, the technique used for blocking the SON and the STN is to palpate the supraorbital notch or supratrochlear notches before local anesthetic administration. The local anesthetic is then injected by inserting the needle perpendicular to the skin along the upper orbital margin[38] around the supratrochlear notch or approximately 1 cm medial to the supraorbital foramen[10] to block the STN. To block the SON, the needle is directed perpendicular to the skin just above the eyebrow line[10,38] at the midpoint of the eyeball in the neutral position or one fingerbreadth lateral to the supratrochlear block.[10,38]
This method is not dependable as there is a high degree of variation in the occurrence of a supraorbital notch/foramen among individuals, as shown in a study done by Fallucco et al. on 60 supraorbital rims on dissection. A supraorbital foramen was present in 26.7%, notch in 83.3%, and both foramen and notch in 10%.[27] In cadavers where both notch and foramen are present, intraconal branching of the SON into deep and superficial branches was present. Similar findings regarding notch and foramen were noted in a study conducted on South Indian skulls by Varsha et al.[39] However, in our study, the incidence of the supraorbital foramen is much higher (17 out of 22) than the presence of a supraorbital notch. These variations require the anesthetist to block the branches separately, which are otherwise blocked at a single point effectively and are missed, leading to inadequate anesthesia. Variations within the same cadaver among the right and left sides were also noted, further complicating the technique of nerve blocks. Furthermore, the supraorbital foramen and supraorbital notch with tiny Type II fascial band (according to the classification of fascial band variations present at the supraorbital notch by Fallucco et al.)[27] may not always be palpable with obesity, and distortion of anatomical landmarks further limiting the ability to locate the SON accurately. The supraorbital foramen/notch is also implicated in migraine and frontal headaches secondary to compression and nerve entrapment.[27,29] Another common method for blocking the two nerves is to measure 2–3 finger breadths distance or 2–3 cm from the midline for supratrochlear and SONs, respectively. However, the fingerbreadths and the skull sizes vary considerably leading to various complications associated with these scalp nerve blocks and hence should be avoided. To overcome the difficulty of specifically measuring the distances from the bony landmarks, we have calculated the ratios for the distances of the supraorbital and STNs from the bony landmarks compared to the distances from standard bony landmarks for each specimen. On finding the ratios, it was noticed that overall, the SON is found approximately midway between the distances from the midline to the LOM. The STN is found approximately at the junction between medial and middle one-third of the distance from the midline to the LOM. The distance from the midline to the SON is about three-fourths the distance between the medial orbital margin and the LOM. The distance from the midline to the STN is about half the distance between the medial orbital margin and the LOM. These findings can be used in clinical practice to provide quicker nerve blocks, especially in emergencies with limited time at hand. Furthermore, these will be customized to each patient as they are based on their skull parameters. The SON is found lateral to the corresponding vessels on both sides in the majority of the specimens. The STN relation to the corresponding vessels could not be made out in the majority of the specimens due to the inability to find the vessels. However, in cases where the vessels are found, the nerve is found lateral to the corresponding vessels on both sides in the majority of the specimens. The majority of the supraorbital and STNs were found emerging through the respective foramina. There is also asymmetry regarding notch and foramen noted between each side of the same cadaver. The majority of the specimens showed SONs giving off three branches and STN not giving any branches within 1 cm from the point of their emergence. The GON is the primary sensory supply for the back of the scalp. For blocking the GON, the occipital artery, whose pulsations can be felt, is palpated about 3–4 cm lateral to the external occipital protuberance along the superior nuchal line, and the local anesthetic drug is injected just medial to the occipital artery.[38] However, the relation of the nerve to the artery is not always medial, as shown in a study done in six infant cadavers by Prigge et al. showed that the occipital artery is found between the branches of the GON in two hemiskulls.[40] In our study, the GON is seen medial to the occipital artery in 63.63% lateral in 18.18% and superficial to the artery in 13.63%. The nerve is located between the bifurcated branches of the occipital artery in 4.54%. These findings further add to the above statement that the nerve is not always medial to the vessel. This can lead to a patchy sensory block, damage to the nerve or the vessel, and inadvertently injecting the anesthetic drug into the vessel causing systemic complications. Other methods include injecting the drug halfway between occipital protuberance and mastoid process 2.5 cm lateral to the nuchal medial line.[10] However, using a specific distance in numbers for all the population may not be practically correct as the skull sizes vary from individual to individual. In our study, inion, the midpoint over the external occipital protuberance and the mastoid process, easily palpable bony prominences were taken as the reference points. A ratio was calculated for the distances measured from GON to these bony landmarks. It was found that the GON is located approximately two-fifths the distance between inion and the mastoid process on the line joining them. The GON pierced the semispinalis capitis muscle and travelled in a superolateral course in all the specimens, with the majority emerging as unsplit. The GON is found to be medial to the occipital artery on the right and lateral to the occipital artery on the left in the majority of the specimens, thus exhibiting asymmetry bilaterally. In the majority of the specimens, GON did not give any branches before the line joining the inion and mastoid.
This is a preliminary study and we tried to provide a baseline for comparison between sides with limited sample number. Definitely, statistical significance cannot be drawn with this limited sample number, which is a limitation for the present study. Thus, follow-up studies with larger sample size can be conducted based on the preliminary findings based on the present study.
CONCLUSION
From our cadaveric study it could be inferred that the effective blockade of the major cutaneous nerves of scalp can be obtained by injecting the local anesthesia as follows.
SON
By injecting the anesthetic drug at a point midway between the midline and the LOM, at the level of the supraorbital margin.
STN
By injecting the anesthetic drug at a point between the medial one-third and lateral two-thirds of the line joining the midline and the LOM, at the level of the supraorbital margin.
GON
A point between the medial two-fifth and lateral three-fifth of the distance of the line joining the inion and the mastoid process would result in the successful block of the GON.
Declaration of patient consent
The authors certify that they have obtained all appropriate consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesth Intensive Care. 2006;34:224-7.
- [CrossRef] [PubMed] [Google Scholar]
- Preemptive analgesia for postoperative pain after frontotemporal craniotomy. No Shinkei Geka. 2002;30:171-4.
- [Google Scholar]
- Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001;93:1272-6.
- [CrossRef] [PubMed] [Google Scholar]
- Anaesthesia for awake craniotomy: A modern approach. J Clin Neurosci. 2004;11:16-9.
- [CrossRef] [PubMed] [Google Scholar]
- Greater occipital nerve block for surgical resection of major infiltrating lesions of the posterior scalp. Plast Reconstr Surg. 2010;125:52.e-3e.
- [CrossRef] [PubMed] [Google Scholar]
- Nerve blocks enable adequate pain relief during topical photodynamic therapy of field cancerization on the forehead and scalp. Br J Dermatol. 2009;160:795-800.
- [CrossRef] [PubMed] [Google Scholar]
- Blocking the greater occipital nerve: Utility in headache management. Curr Pain Headache Rep. 2010;14:404-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosurgical considerations and general methods for craniotomy under local anesthesia. Int Anesthesiol Clin. 1986;24:89-114.
- [CrossRef] [PubMed] [Google Scholar]
- "Scalp block" during craniotomy: A classic technique revisited. J Neurosurg Anesthesiol. 2010;22:187-94.
- [CrossRef] [PubMed] [Google Scholar]
- Sensory complications in patients after scalp mass excision and its anatomical considerations. J Korean Neurosurg Soc. 2014;55:200-4.
- [CrossRef] [PubMed] [Google Scholar]
- Transient facial nerve palsy after the scalp block for burr hole evacuation of subdural hematoma. Turk J Anaesthesiol Reanim. 2018;46:238-40.
- [CrossRef] [PubMed] [Google Scholar]
- Unilateral complete ptosis after scalp block for awake craniotomy: A rare complication. J Neuroanaesthesiol Crit Care. 2018;5:111-3.
- [CrossRef] [Google Scholar]
- The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int. 2011;2:178.
- [CrossRef] [PubMed] [Google Scholar]
- Branching pattern of mental nerves in south Indian population: A cadaveric dissection study. Indian J Clin Anat Physiol. 2020;5:540-5.
- [CrossRef] [Google Scholar]
- An anatomical study of the nerves targeted for sensory blocks of the head and neck in neonates and infants South Africa: University of Pretoria; 2018. p. :232.
- [Google Scholar]
- Variations of the frontal exit of the supraorbital nerve: An anatomic study. Plast Reconstr Surg. 1998;102:334-41.
- [CrossRef] [PubMed] [Google Scholar]
- Variations in the anatomy of the auriculotemporal nerve. Clin Anat. 2005;18:15-22.
- [CrossRef] [PubMed] [Google Scholar]
- Supraorbital notch and foramen: Positional variation and relevance to direct brow lift. Ophthalmic Plast Reconstr Surg. 2013;29:67-70.
- [CrossRef] [PubMed] [Google Scholar]
- The anatomy of the corrugator supercilii muscle: Part II. Supraorbital nerve branching patterns. Plast Reconstr Surg. 2008;121:233-40.
- [CrossRef] [PubMed] [Google Scholar]
- The zygomaticotemporal branch of the trigeminal nerve: Part II. Anatomical variations. Plast Reconstr Surg. 2010;126:435-42.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical variations of the supraorbital and supratrochlear nerves: Their intraorbital course and relation to the supraorbital margin. Med Sci Monit. 2019;25:5201-10.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical consideration of the anterior and lateral cutaneous nerves in the scalp. J Korean Med Sci. 2010;25:517-22.
- [CrossRef] [PubMed] [Google Scholar]
- The frontotemporal peripheral nerves. Topographic variations of the supraorbital, supratrochlear and auriculotemporal nerves and their possible clinical significance. Surg Radiol Anat. 2001;23:97-104.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization and localization of the supraorbital and frontal exits of the supraorbital nerve in Chinese: An anatomic study. Ophthalmic Plast Reconstr Surg. 2006;22:209-13.
- [CrossRef] [PubMed] [Google Scholar]
- Variations in the anatomy of the posterior auricular nerve and its potential as a landmark for identification of the facial nerve trunk: A cadaveric study. Anat Sci Int. 2012;87:101-5.
- [CrossRef] [PubMed] [Google Scholar]
- The anatomical morphology of the supraorbital notch: Clinical relevance to the surgical treatment of migraine headaches. Plast Reconstr Surg. 2012;130:1227-33.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical variations of the occipital nerves: Implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009;123:859-63.
- [CrossRef] [PubMed] [Google Scholar]
- Supraorbital Rim Syndrome: Definition, surgical treatment, and outcomes for frontal headache. Plastic and reconstructive surgery. Global Open. 2016;4:e795.
- [CrossRef] [PubMed] [Google Scholar]
- A study of the supraorbital nerve. Plast Reconstr Surg. 1995;96:564-9.
- [CrossRef] [PubMed] [Google Scholar]
- A case of occipital neuralgia in the greater and lesser occipital nerves treated with neurectomy by using transcranial doppler sonography: Technical aspects. Korean J Pain. 2011;24:48-52.
- [CrossRef] [PubMed] [Google Scholar]
- MR neurographic evaluation of facial and neck pain: Normal and abnormal craniospinal nerves below the skull base. Radiographics. 2018;38:1498-513.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy, head and neck, occipital nerves Treasure Island (FL): StatPearls Publishing; 2021.
- [Google Scholar]
- The anatomy of the greater occipital nerve: Part II. Compression point topography. Plast Reconstr Surg. 2010;126:1563-72.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: A comparison of two selective techniques confirmed by anatomical dissection. Br J Anaesth. 2010;104:637-42.
- [CrossRef] [PubMed] [Google Scholar]
- Schwannoma of the greater occipital nerve: An uncommon cause of occipital neuralgia. J Neurosci Rural Pract. 2015;6:634-6.
- [CrossRef] [PubMed] [Google Scholar]
- Anaesthesia for awake craniotomy. Contin Educ Anaesth Crit Care Pain. 2014;14:6-11.
- [CrossRef] [Google Scholar]
- Incidence and morphological study of supraorbital foramen in South Indian skulls. J Pharm Sci. 2015;7:3.
- [Google Scholar]
- Anatomy of the greater occipital nerve block in infants. Paediatr Anaesth. 2019;29:945-9.
- [CrossRef] [PubMed] [Google Scholar]






