Translate this page into:
A randomized controlled trial of brief intervention for patients with cannabis use disorder
*Corresponding author: Dr. Siddharth Sarkar, Department of Psychiatry and NDDTC, All India Institute of Medical Science, New Delhi, Delhi, India. sidsarkar22@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shekhawat AS, Mathur R, Sarkar S, Kaloiya GS, Balhara Y. A randomized controlled trial of brief intervention for patients with cannabis use disorder. J Neurosci Rural Pract 2023;14:710-6.
Abstract
Objectives:
Effective interventions for cannabis use disorders are fairly limited. The present randomized controlled trial (RCT) aimed to compare the reduction in cannabis use (number of days cannabis used) with brief intervention and simple advice in patients with cannabis use disorder.
Materials and Methods:
This non-blinded and parallel two-group RCT included 100 male patients with cannabis use disorder. A semi-structured pro forma and severity of dependence scale (SDS) were used. Participants were then randomized to either of the two arms (brief intervention and simple advice) in a 1:1 ratio. Cannabis use patterns and SDS scores were assessed over the phone at week 4, week 8, and week 12.
Results:
The two groups were comparable in sociodemographics and cannabis use characteristics. Participants in both groups were using cannabis for 30 days in the past month before enrolment. The brief intervention group had a lesser number of days of cannabis use vis-a-vis the simple advice group at 4, 8, and 12 weeks. There was a significant time effect for change in SDS scores (F = 30.629, P < 0.001), but the group effect was not significant (F = 0.379, P = 0.541).
Conclusion:
In this population of regular cannabis users, brief intervention may be useful in reducing cannabis usage. It can be integrated into routine assessments and management of those with regular use of cannabis.
Keywords
Cannabis
Brief intervention
Randomized controlled trial
INTRODUCTION
Cannabis is by far the most extensively cultivated, seized, and abused illicit substance in the world.[1] In the year 2019, about 200 million individuals used cannabis globally.[1] Cannabis usage over many years and decades appears to cause long-term memory and cognition problems, particularly when cannabis use is initiated early.[2,3]
Major epidemiological studies have also revealed the high frequency of cannabis use in different countries. The National Epidemiologic Survey of Alcohol and Related Conditions–III found that cannabis usage among adults doubled between the early 2000s and 2010s, which also reflected a substantial increase in the prevalence of cannabis use disorders.[4] According to the most recent National Survey on Drug Use and Health, current cannabis use in the United States (in the previous month) grew from 31.6 million to 32.8 million in 2021. In Australia, Europe, and the United States, the number of individuals with cannabis-related problems who seek treatment has increased during the last couple of decades.[5-7]
There are currently very limited pharmacological agents licensed for the management of cannabis use disorder. Although several pharmacologic treatments have been suggested for off-label use, there is insufficient evidence to guide clinical practice based on studies conducted to date on pharmacotherapies for cannabis dependence.[8]
Only a few individuals with cannabis use disorder seek treatment.[9] According to reports, about 10–36% of people with a cannabis use disorder get therapy,[10-12] while the rest go untreated. Despite its widespread use and significant morbidity, no pharmacological treatment for addiction to cannabis is currently authorized. Hence, psychosocial approaches remain the mainstay for addressing this issue. Various systematic reviews and meta-analyses have highlighted that individuals with cannabis use disorder who received a psychosocial intervention fared better.[13,14] The evidence base of approaches for cannabis use disorders is gradually expanding. Cognitive behavioral techniques, contingency management, motivation improvement approaches, and a variety of brief interventions are just a few of them. Many interventions aimed at those seeking therapy are intensive in terms of time and money required, and their availability is typically limited. Brief interventions have been designed to address the issue of time and resource commitments required. Brief intervention is a systematic, non-judgmental, and client-centered treatment delivered by a trained individual over the course of 1–4 counseling sessions (each session usually 5–30 min).[15-17] Hence, they could be implemented to help many people with cannabis use disorder and related issues. Cannabis cessation rates have been observed to improve when brief psychological therapies based on motivational interviewing approaches are used.[18] The findings of research evaluating the efficacy and/ or effectiveness of limited-session therapies for the treatment of cannabis use disorders suggest that there can be a variable extent of improvements in outcome measures compared to no therapy.[19,20]
In the Indian setting, according to a recent pan-Indian national level survey, more than 3% of adults and about 1% of adolescents used cannabis in the preceding year.[21] Overall, men (5%) were more likely than women (0.6%) to have used cannabis in the previous year. Balhara et al.[22] examined cannabis use trends and found that around 14% of those seeking treatment for addictive disorders reported cannabis use. Polysubstance users were common in this sample. In India, cannabis use is rarely the presenting complaint of treatment-seekers.[23] Hence, there is a need to more comprehensively address cannabis use disorder.
In India where resources are limited and competing public health demands predominate, brief interventions offer some distinct advantages. With limited resources, brief interventions could be an efficient strategy to address the problem of cannabis usage in a wide segment of the population. Furthermore, it has been found that the majority of cannabis users suffer from mild-to-moderate dependence and so are suitable for brief interventions. There are limited studies for brief interventions in the Indian context for cannabis use disorders.[24] Therefore, this study was planned to conduct a single-session outpatient brief intervention for cannabis use disorder. The main objective of the study was to compare the reduction in the days of cannabis use with a manualized brief intervention and simple advice in patients with cannabis use disorder. Another objective was to compare the severity of cannabis use with the two interventions in this population.
MATERIALS AND METHODS
Setting and participants
This was an open, two-group, parallel, and randomized controlled trial (RCT). The study setting was the outpatient department of a dedicated addiction treatment center which is attached to a public-funded medical school in India. A referral is not required for seeking treatment. Treatment is largely subsidized and is provided by a team of clinicians and other ancillary staff. The center largely follows a medical model of care, and treatment is provided through inpatient or outpatient services. Patients with opioid and/or alcohol use disorders generally seek treatment at the center. Many of the patients have additional substance use disorders.
The study included patients aged between 18 and 60 years, who had taken cannabis for at least 14 days in the past 1 month as per self-report, and who met diagnostic and statistical manual of mental disorders (DSM)-5 criteria for mild or moderate cannabis use disorder. Participants were excluded if they were planned for inpatient treatment, had intoxication or severe withdrawal, or had significant medical and/or psychiatric illnesses that were not conducive for an interview.
For the sample size calculation, data from Stephens et al.[25] are used where the population was using cannabis for a mean of 5.76 days/week. The estimation of sample size has been done on the basis reduction number of days of cannabis use in the intervention group at 6 months (4.90 days/week). Based on the above estimates, the required sample size with 80% power and alpha of 0.05 would be 40 in each group (i.e., 80 total). Due to the expected attrition rate of around 25%, the total number of patients has been kept at 50 in each group (i.e., net sample size of 100).
Procedure
Participants who fulfilled the selection criteria were recruited into the study after taking due informed consent. A detailed assessment of the subjects recruited into the study was performed on the 1st day only after taking written informed consent. A semi-structured pro forma developed specifically for this study included the sociodemographic profile, drug use history, pattern and type of cannabis use, medical history, and list of medicines currently being taken and treatment of other concurrent substance use disorders. Included participants were also administered the severity of dependence scale (SDS). SDS includes five measures, all of which are specifically focused on impaired drug control and preoccupation with drug use. The scale has shown good test-retest reliability (intraclass correlation coefficient = 0.88) and internal consistency (alpha = 0.83) in a sample of cannabis users.[26]
The patients were randomized to either of the study arms by simple random allocation (1:1). Computerized random sequence was generated by one of the co-authors and allocated into group A (Brief Intervention) or B (Simple Advice). Opaque envelopes were prepared, labeled sequentially, with a random group (A or B) written on a piece of paper. At the time of randomization (day 1), the allocation was done after opening the sealed envelope, and patients were either allocated into brief intervention or the simple advice group.
Simple advice group patients were encouraged to try to quit cannabis and were advised to continue their efforts. They were given a drug information pamphlet (in English and Hindi). The provision of information was standardized using a script. The patient was told that an inquiry would be made about his cannabis use pattern over the phone at week 4, week 8, and week 12 and that two phone numbers will be recorded.
The brief intervention (psychological intervention) was done in a single session by a trained psychiatrist (AS). The duration was supposed to range from 30 to 90 min. It was conducted in the outpatient setting. The focus was on cannabis use disorder. One family member was allowed in the session based on counselor discretion and the comfort levels of the patient. A patient requiring urgent medical/psychiatric help during or after the intervention for any reason was to be referred to the treating clinician as needed (e.g., a patient expressing suicidal ideation). The key components of this manual-based brief intervention included providing feedback, setting responsibility, advising for change, giving a menu of options for change, expressing empathy toward participants, and enhancing self-efficacy for making the change. The manual developed contains information on the delivery of brief intervention on the basis of principles for motivational interviewing, motivational enhancement, and cognitive behavior therapy. Issues such as the format of the session, the patient-therapist relationship, the structure of the session, and addressing cannabis-related cognitions and behaviors are discussed and presented in the manual. The therapist had the liberty to use the principles mentioned in the manual, but conduct the session based on the circumstances of the individual case. The manual is available at https://sites.google.com/view/enddtc-aiims/resources/manual-for-bi. The pamphlet is available in supplementary material. Five sessions were recorded and transcribed. The content of the sessions was assessed for fidelity by experts.
After 4 weeks, 8 weeks, and 12 weeks of the individual session, patients were contacted telephonically to collect information about the pattern of cannabis use (days of cannabis use in the past month) and severity of dependence.
Clinical Trials Registry-India registration was done (CTRI) (CTRI/2020/12/030067). The Institutional Ethics Committee approved the study in December 2020. Patients were recruited for the study from 12 March 2021 to December 2021.
Statistical analysis
Kolmogorov–Smirnov test checked normality of the data. Basic demographic and clinical data were represented with descriptive statistics. Inferential statistics in the form of group comparisons used Student’s t-test/Mann–Whitney U, analysis of variance, or Kruskal–Wallis test as appropriate. All data were evaluated with the principle of intention-to-treat analysis. Missing data imputation was done using the last observation carried forward method. The days cannabis used in the past 30 days (the primary objective) were compared statistically using the Friedman Test and the Kruskal–Wallis test as the data were non-normal. The SDS scores were compared using a two-way repeated measures test. The data were entered, managed, and screened using Microsoft Excel Spreadsheet, and further, statistical analysis was performed on SPSS software. A P value of less than 0.05 was considered significant. The CONSORT guideline was used for reporting the results of the study.
RESULTS
One hundred and thirty-five participants were approached for inclusion. Out of them, 35 were excluded (31 did not fulfill the criteria of mild-to-moderate cannabis use disorder, two were <18 years, and two refused participation). Thus, 100 participants were randomized to either of the two arms (brief intervention and simple advice). There were no significant differences between both arms in terms of their sociodemographic profile [Table 1]. The CONSORT diagram is provided in Figure 1.
| Socio-demographic characteristics | Brief intervention group (n=50) | Simple advice group (n=50) | P-value |
|---|---|---|---|
| Age (years) | 26 (22–29) | 24.5 (22–33) | 0.953 |
| Gender | |||
| Male | 50 (100%) | 50 (100%) | NA |
| Marital status | |||
| Married | 26 (52%) | 19 (38%) | 0.159 |
| Unmarried | 24 (48%) | 31 (62%) | |
| Education | |||
| <10thgrade | 36 (72%) | 43 (86%) | 0.086 |
| More than 10thgrade | 14 (28%) | 7 (14%) | |
| Current employment status | |||
| Not employed currently | 15 (30%) | 18 (36%) | 0.523 |
| Currently employed | 35 (70%) | 32 (64%) | |
| Age at first use of Cannabis (years) | 17 (15–20) | 17.5 (15–20.75) | 0.748 |
| Severity of dependence scale score | 9.84±2.18 | 9.70±2.31 | 0.756 |
| Number of days of use of cannabis in last month | 30 (30–30) | 30 (30–30) | 0.318 |
| Comorbid substance use disorder | |||
| Tobacco use disorder ever | 49 (98%) | 50 (100%) | 1.000 |
| Alcohol use disorder ever | 4 (8%) | 5 (10%) | 1.000 |
| Opioid use disorder ever | 46 (92%) | 46 (92%) | 1.000 |
| Sedative hypnotics use disorder ever | 4 (8%) | 5 (10%) | 1.000 |
| Injecting drug use ever | 7 (14%) | 13 (26%) | 0.134 |
| Ever admitted for treatment of substance use disorder | 6 (12%) | 3 (6%) | 0.487 |
Shown as median (inter-quartile range) or frequency (percentage), comparison done using Independent t-test, Mann–Whitney test, Fisher’s exact test or Chi-square test as applicable

- CONSORT diagram of the trial.
The two groups did not diverge in terms of the type of cannabis use. Neither did the groups vary substantially in terms of location of cannabis use, time of day cannabis intake, and cannabis used alone or with someone else. While a majority of the participants had comorbid opioid use disorder, the two groups were not different in terms of the use of other substances including tobacco, alcohol, opioid, and benzodiazepines.
At baseline, past-month cannabis use was similar across the two groups. The median number of days of past-month cannabis use at baseline in the brief intervention group and simple advice group was 30, suggesting that participants were consuming cannabis daily. The median number of past month cannabis use in brief intervention groups were 25, 26.5, and 30 days at 4, 8, and 12 weeks, respectively. The same figures for the simple advice group were 30 days each at 4, 8, and 12 weeks. The details are presented in Table 2 and diagrammatically represented in Figure 2.
| Time point comparison (Change in number of days of Cannabis use in past month) | Brief intervention group (n=50) |
Simple advice group (n=50) |
P-value (comparison of Brief intervention and simple advice based on Mann Whitney U-test) |
|---|---|---|---|
| Baseline | 30 (30–30) | 30 (30–30) | 0.318 |
| 4 weeks | 25 (22.25–30) | 30 (28–30) | <0.001* |
| 8 weeks | 26.5 (25–30) | 30 (30–30) | 0.002* |
| 12 weeks | 30 (26.5–30) | 30 (30–30) | 0.049* |
| Intra group P value (based on Friedman test) | <0.0001* | 0.0003* |
Shown as median (inter-quartile range), *P<0.05
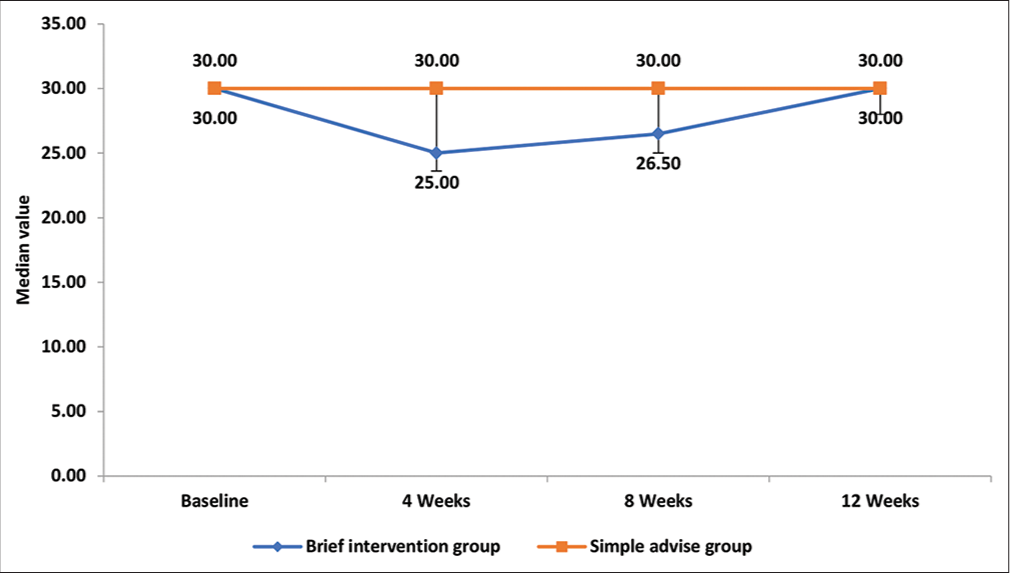
- Comparison of number of days of cannabis use in past month at different time intervals between brief intervention and simple advice group.
The intragroup comparison showed that there were significant changes in scores at various assessment points in both groups. The intergroup comparison demonstrated a significant difference in the number of days of past-month cannabis use at 4, 8, and 12 weeks between the brief intervention and simple advice group (lower in the brief intervention group when compared with the simple advice group, P < 0.001, 0.002, and 0.049 at 4, 8, and 12 weeks, respectively).
The mean score of SDS at baseline, 4, 8, and 12 weeks in both groups is shown in Table 3. The intragroup comparison for the brief intervention group and simple advice group showed there was a significant reduction in SDS scores at various assessment points in both groups. The intergroup comparison for the brief intervention group and simple advice group found a significant interaction (between time and group), but group interaction was not significant, suggesting the present sample was not able to demonstrate a specific decrease in SDS scores attributable to the brief intervention.
| SDS | Brief intervention group (n=50) | Simple advice group (n=50) | Time effect | Group effect | Time X group effect |
|---|---|---|---|---|---|
| Baseline | 9.84±2.18 | 9.70±2.31 | F=30.629; | F=0.379; | F=9.879; |
| 4 weeks | 8.66±2.11 | 9.30±2.51 | df=1, | df=1, | df=1, |
| 8 weeks | 8.82±2.10 | 9.44±2.53 | P<0.001* | P=0.541 | P=0.003* |
| 12 weeks | 9.54±2.23 | 9.56±2.39 | |||
| Intra group P-value | F=23.829; df=1, P<0.001* | F=8.173; df=1, P=0.006* |
Shown as Mean±Standard Deviation, *P<0.05. SDS: Severity of dependence scale
No patient in either of the groups achieved complete abstinence from cannabis use at any of the time periods.
DISCUSSION
This study found that those individuals receiving one session of brief intervention had more reduction in the number of days of use of cannabis than those receiving simple advice. In the brief intervention group, the mean absolute percentage change in cannabis use days in the past month at 4 weeks was 12.5%, at 8 weeks about 9.3%, and at 12 weeks about 3.8%.
These findings are comparable to the previous studies. A RCT by Copeland et al.,[27] conducted with treatment-seeking adults found that one session of cognitive behavioral therapy intervention (equivalent to a brief intervention session) showed a trend of a greater proportion of days of not using cannabis than a delayed treatment control group. Similarly, the study conducted by Marijuana Treatment Project Research Group.[20] which evaluated the outcomes of two brief interventions for cannabis-dependent adults showed a greater reduction in the marijuana smoking days with two-session and nine-session treatments than delayed treatment control condition.
This study also compared the SDS scores at 4, 8, and 12 weeks across brief intervention and simple advice groups. There was a significant difference in SDS scores across the two groups over time. The decline in SDS scores in the two groups was comparable. These findings are comparable to the previous literature.[28] This could be attributable to the fact that changes were minimal in both the groups and possibly simple advice might have resulted in some behavioral “impact.”
There were no participants in either group who were abstinent from cannabis use. This finding was contrary to what is reported in the previous literature.[19,28] The explanation for this divergence could be participants using cannabis daily in the present study (compared to occasional users in the previous studies), and single session brief intervention being used in the present study.
The implications of the present study are several. One, brief intervention is a time-limited intervention approach that is scalable. Implementation of brief interventions is easier in a busy clinical setting like ours with high patient loads and limited time per patient. Second, a manualized brief intervention may be conducted by several types of mental health professionals during routine clinical encounters. The manual developed for the clinicians is available from the authors on request. Third, brief interventions for addressing cannabis use may be applicable for patients with other substance use disorders (mainly opioids).
A few limitations should be noted about the study. First, participants who were seeking help were selected; thus, the results may not generalize to persons who are unmotivated to seek treatment. Second, the study was conducted in the outpatient setting at a tertiary care center, and hence, it is difficult to comment on if the findings would be generalizable to patients with cannabis use disorder seeking treatment at other settings, such as community outreach clinics, or primary care centers. Third, the study was based on self-report of cannabis use, and no other measures such as urine screening or corroboration with collateral sources were used to confirm the cannabis use. Another limitation can be that this study was non-blinded which may have resulted in favorable outcomes for the intervention group. Furthermore, the presence of a high proportion of co-occurring substance use in both groups might have influenced some findings. Finally, this was based only on a single session delivered by a single professional. Multi-session intervention packages may have different results.
CONCLUSION
Despite the limitations, the present study suggests that brief intervention may help in reducing cannabis use among those with mild to moderate cannabis use disorder and using other substances. Regularly directed brief interventions to patients entering the treatment process may help to reduce cannabis usage. In the future, studies can be conducted to explore how the delivery of intervention could be improved, assess the impact of delivery by different professionals, and evaluate the impact of different substances on the outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- World Drug Report 2021. United Nations: Office on Drugs and Crime; Available from https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html [Last accessed on 2022 Dec 21]
- [Google Scholar]
- Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-64.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-27.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-42.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology of cannabis use and cannabis-related harm in Australia 1993-2007. Addiction. 2010;105:1071-9.
- [CrossRef] [PubMed] [Google Scholar]
- Atlas on Substance Use (2010): Resources for the Prevention and Treatment of Substance Use Disorders Geneva: World Health Organization; 2010.
- [Google Scholar]
- New Developments in Europe's Cannabis Market (Perspectives on Drugs) Available from: https://www.emcdda.europa.eu/publications/pods/cannabis-markets-developments_en [Last accessed on 2022 Dec 21]
- [Google Scholar]
- World Drug Report (United Nations Publication Sales No. E.19.XI.8) 2019. Available from: https://wdr.unodc.org/wdr2019/prelaunch/wdr19_booklet_1_executive_summary.pdf [Last accessed on 2023 Mar 23]
- [Google Scholar]
- Prevalence and correlates of treatment utilization among adults with cannabis use disorder in the United States. Drug Alcohol Depend. 2017;177:153-62.
- [CrossRef] [PubMed] [Google Scholar]
- Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28:643-52.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabis use disorders in the USA: Prevalence, correlates and co-morbidity. Psychol Med. 2006;36:1447-60.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence, correlates and comorbidity of DSM-IV cannabis use and cannabis use disorders in Australia. Aust N Z J Psychiatry. 2012;46:1182-92.
- [CrossRef] [PubMed] [Google Scholar]
- Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;2016:CD005336.
- [CrossRef] [Google Scholar]
- Brief interventions for cannabis use in emerging adults: A systematic review, meta-analysis, and evidence map. Drug Alcohol Depend. 2019;204:107565.
- [CrossRef] [PubMed] [Google Scholar]
- Brief interventions for alcohol problems: A meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279-92.
- [CrossRef] [PubMed] [Google Scholar]
- Motivational enhancement and other brief interventions for adolescent substance abuse: Foundations, applications and evaluations. Addiction. 2004;99(Suppl 2):63-75.
- [CrossRef] [PubMed] [Google Scholar]
- Motivational Interviewing: Helping People Change New York City: Guilford Press; 2012.
- [Google Scholar]
- Overview Drug misuse in over 16S: Psychosocial Interventions Guidance NICE. Available from: https://www.nice.org.uk/guidance/cg51 [Last accessed on 2022 Dec 21]
- [Google Scholar]
- Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898-908.
- [CrossRef] [PubMed] [Google Scholar]
- Brief treatments for cannabis dependence: Findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455-66.
- [CrossRef] [PubMed] [Google Scholar]
- Magnitude of Substance Use in India New Delhi: Ministry of Social Justice and Empowerment, Government of India; 2019.
- [Google Scholar]
- Time trends of cannabis use among treatment-seeking individuals at Government de-addiction centers across India over a period of 7 years. Indian J Psychol Med. 2016;38:331-5.
- [CrossRef] [PubMed] [Google Scholar]
- An exploratory study of cannabis use pattern and treatment seeking in patients attending an addiction treatment facility. Indian J Psychiatry. 2020;62:145-51.
- [CrossRef] [PubMed] [Google Scholar]
- Screening and brief intervention for cannabis misuse in individuals on buprenorphine treatment for opioid use disorder: Double-blind randomized clinical trial. J Psychoactive Drugs 2022:1-10. doi: 10.1080/02791072.2022.2143458. Epub ahead of print
- [CrossRef] [PubMed] [Google Scholar]
- The Marijuana Check-up: Promoting change in ambivalent marijuana users. Addiction. 2007;102:947-57.
- [CrossRef] [PubMed] [Google Scholar]
- The Severity of Dependence Scale (SDS) in an adolescent population of cannabis users: Reliability, validity and diagnostic cut-off. Drug Alcohol Depend. 2006;83:90-3.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat. 2001;21:55-64. discussion 65-6
- [CrossRef] [PubMed] [Google Scholar]
- Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051-61.
- [CrossRef] [PubMed] [Google Scholar]







