Translate this page into:
Sleep quality evaluation, correlation with headache frequency, and propensity to conversion from episodic to chronic daily headache in migraine patients: A cross-sectional study
*Corresponding author: Sanjeev Kumar Bhoi, Department of Neurology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. sanjeev_bhoi@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bag A, Bhoi SK, Jha M, Palo GD. Sleep quality evaluation, correlation with headache frequency, and propensity to conversion from episodic to chronic daily headache in migraine patients: A cross-sectional study. J Neurosci Rural Pract 2023;14:70-7.
Abstract
Objective:
The aim of the study was to determine the association between sleep quality with headache frequency in migraine patients and also to evaluate migraine trigger and non-headache symptoms in episodic and chronic migraine groups and evaluation of the same in poor and good sleepers (GSs) in migraine population.
Materials and Methods:
In a cross-sectional and observational study in a tertiary care hospital of East India between January 2018 and September 2020, migraine patients were evaluated. Migraine patients were divided into two groups: Episodic migraine (EM) and chronic migraine (CM) group according to ICHD 3 b classification and into poor sleepers (PSs, Global Pittsburgh Sleep Quality Index [PSQI] >5) and GSs (Global PSQI ≤5). Sleep was evaluated using PQSI – a self-rated questionnaire and disease pattern, associated non-headache symptoms, and triggers were evaluated in between groups. Demographic, headache character, and sleep parameters including seven “component” scores: Subjective sleep quality, sleep latency, sleeps duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction and global PQSI were compared between groups (EM and CM). Similar parameters were also compared between PSs and GSs group. Statistical analysis was performed using the χ2 test for categorical variables and the t-test and Wilcoxon rank-sum test for continuous variables. Correlation between two normally distributed numerical was tested by Pearson correlation coefficient assessment.
Results:
One hundred migraine patients were analyzed, among which 57 were PSs and 43 were GSs and 51 patients had EM and 49 patients had CM. Moderately significant “r” value noted in between headache frequency and global PQSI score (r = 0.45, P < 0.01). In non-headache symptoms, blurring of vision (EM 8 [16%] and CM 16 [33%] P = 0.05), nasal congestion (EM – 3 [6%] and CM – 12 [24%], P = 0.01), and cervical muscle tenderness (EM– 23 [45%] and CM – 34 [69%], P = 0.01) were more prevalent in chronic headache group along with allodynia (EM – 11 [22%] and CM – 25 [51%], P < 0.01).
Conclusion:
Chronic headache group had poor subjective sleep quality, increased sleep latency, decreased sleep duration, decreased sleep efficiency, and increased sleep disturbance in comparison to episodic group which has therapeutic implication. The non-headache symptoms which are more prevalent in CM patients increase the overall disability.
Keywords
Sleep
Allodynia
Migraine
Non-headache symptoms
Triggers
INTRODUCTION
Global burden of disease study 2017 has put headache disorders mainly migraine as the second leading cause of disability worldwide[1] with a 1-year period prevalence of 11.7% (17.1% in women and 5.6% in men).[2] Migraine-related disability clearly depends on recurrence of attacks, headache severity, and duration of headache. After introduction of 4-phase pathophysiology of migraine, it became apparent that disability also depends on multiple associated non-headache symptoms in premonitory and postdrome phase.[3] Headache which occurs for 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days[4] per month and not better explained by other etiology is considered as chronic migraine (CM) according to ICHD 3 beta classification. Recent articles suggest that high-frequency episodic migraine with monthly headache days of 8 or more has disability comparable with CM.[5] Predictors of frequent relapse and chronicity identification remained research target since decades.
Large-scale epidemiological studies have documented sleep disorders up to 20–30% of general population which tend to increase with ageing and more prevalent in developed countries.[6] Poor sleep quality along with chronically short sleep pattern such as insomnia, “awakening headache,” or headache that disrupts sleep was earlier also documented to affect headache severity and frequency in migraineurs.[7] Earlier few study documented half of migraineurs reports difficulty in initiating and maintaining sleep at night occasionally and over one-third reports it to happen even frequently.[7-9] We, in our study, assessed the sleep quality in episodic and chronic migraineurs and also compared triggers and non-headache symptom in these two groups to identify any potential reversible factors to reduce headache day loss. We also tried to find out whether any correlation exists between headache frequency and sleep quality, disease duration, triggers, and non-headache symptoms.
MATERIALS AND METHODS
The study was conducted between January 2018 and September 2020 in a tertiary care teaching hospital in East India. This was a cross-sectional and observational study conducted on outpatient basis. We included patients of ≥18 years of age with history and clinical examination suggestive of primary headache and migraine (ICHD 3 b classification). Patient’s history suggestive of secondary headache and painful cranial neuropathies and other headache and facial pain were excluded from the study. Pregnant ladies and patient having sleep-related breathing disorder, central disorder of hypersomnolence, Parasomnia, and sleep-related movement disorder (International Classification of Sleep Disorder-Third Edition AASM) were excluded from the study. Furthermore, patients on prior therapy with any regular sedative medications and migraine prophylaxis medication in the past 3 months were excluded from the study. The study was approved by the Institutional Ethics Committee (T/IM-NF/Neuro/17/48) and informed consent was obtained from patients fulfilling the inclusion and exclusion criteria and also willing to give consent.
A research questionnaire was formulated to evaluate demographic details, headache character, and sleep quality in each patient. All the patients underwent detailed neurological evaluation and investigation to establish diagnosis and exclude secondary headache. Detailed history regarding duration of illness, duration of headache in each episode, onset to peak time, aura, trigger factors for headache, distribution of headache, associated non-headache symptoms such as blurring of vision, vertigo, diplopia, scotoma, periorbital pain, neck pain, nausea, vomiting, cervical muscle tenderness, conjunctival redness, nasal congestion, hemisensory pain, tired feeling, difficulty in concentration during either headache phase or in postdrome phase, and medication history were noted.
Sleep quality in each patient was evaluated using the Pittsburgh Sleep Quality Index (PQSI) – a self-rated questionnaire which assesses sleep quality and disturbances. This 19 question proforma with seven “component” scores: Subjective sleep quality, sleep latency, sleeps duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction scores were obtained from participating patients. The sum of scores for these seven components yields one global score (global PQSI). The Epworth Sleepiness Scale was used to assess consequence of sleep disturbances in patient’s daily activity. The total score is based on a scale of 0–24 which estimates whether migraineurs are experiencing excessive sleepiness that possibly requires medical attention or hampering activities of daily living.
PQSI is widely used tool in clinical practice and as research tool for sleep quality assessment. Earlier landmark study by Buysse et al.[10] documented that in distinguishing good and poor sleepers (PS), a global PSQI score >5 yields a sensitivity of 89.6% and a specificity of 86.5%. Study population was divided into two groups into PSs (global PSQI >5) and good sleepers (GSs, global PSQI ≤5). Demographic characteristics, duration of illness, headache frequency, headache characters, migraine triggers, and associated non-headache symptoms were compared between the two groups.
Migraine patients were again divided into two group’s EM and CM group according to ICHD 3 b classification.[4] Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month considered as CM after ruling out other possible etiology and secondary causes. Total headache frequency of the past 3 months was taken into account for classifying the patients as chronic or EM patient. Demographic characters, headache character, and sleep parameters including seven “component” scores: Subjective sleep quality, sleep latency, sleeps duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction and Global PQSI were compared between groups.
The primary objective was to determine the association of sleep quality with headache frequency. Secondary objective was to identify whether any specific sleep character correlates better with propensity of conversion from episodic headache to chronic form. We also evaluated whether there is any change in disease pattern, associated non-headache symptoms, and triggers of migraine in between good versus PSs.
Statistical analysis
The analysis was conducted in SPSS version 26. Statistical analysis was performed using the χ2 test for categorical variables and the t-test and Wilcoxon rank-sum test for continuous variables. Continuous variables not following standard distribution were analyzed with the Mann–Whitney U-test. Values in absolute numbers and in percentages were compared between two groups. Results were considered to be statistically significant if P value was < 0.05. Shapiro– Wilk test (P > 0.05) and visual inspection of histogram with Q-Q plots and box plots were used to assess the distribution of variables. Correlation between two normally distributed numerical was tested by Pearson correlation coefficient assessment.
RESULTS
A total of 219 patients were screened and 113 patients were excluded according to exclusion criteria. Among them, 63 patients had tension-type headache, 50 patients were excluded because of ongoing prophylactic medications or both. A total of 106 patients were included during the study period and six patients were excluded as five denied for consent and one patient later diagnosed as idiopathic intracranial hypertension. Data analyzed for total of 100 patients among which 57 were PSs and 43 were GSs. Among the 100 patients, 51 patients had EM and rest 49 patients had CM.
Mean age of migraine patients was 31.83 ± 9.63 years (range 18–60), with female: male ratio 2.7:1 (male 27 [27%] and female 73 [73%]). The mean duration of illness was 7.07 ± 5.40 years (range 1–25). Patients fulfilling the criteria of EM were 51 (51%) and 49 (49%) patients were included as CM [Table 1]. According to global PSQI, 57 (57%) patients were included in poor sleeper group and 43 (43%) were included in good sleeper group. Migraine trigger analysis was done depending on 19 common triggers according to the previous studies.[11] On trigger analysis, physical stress 44 (44%), mental stress in 53 (53%), fasting in 23 (23%), hunger 29 (29%), sun exposure 82 (82%), noise 49 (49%), cold 16 (16%), hot 20 (20%), weather change 22 (22%), post-coital 2 (2%), sleep deprivation 42 (42%), perfume 16 (16%), menstruation 10 (10%), food 8 (8%), fatigue 1 (1%), eye strain 7 (7%), hair wash 23 (23%), dehydration 5 (5%), and exercise in 8 (8%) patients were documented [Table 1].
| Age mean in years (SD, range) | 31.83 (9.63, range 15–60 years.) |
|---|---|
| Gender, number (%) | |
| Female | 73 (73) |
| Male | 27 (27) |
| Presence of migraine triggers (% of study population reported as trigger factor) | |
| Physical stress | 44 (44) |
| Mental stress | 53 (53) |
| Fasting | 23 (23) |
| Hunger | 29 (29) |
| Sun exposure | 82 (82) |
| Noise | 49 (49) |
| Cold | 16 (16) |
| Hot | 20 (20) |
| Weather change | 22 (22) |
| Post-coital | 2 (2) |
| Sleep deprivation | 42 (42) |
| Perfume | 16 (16) |
| Menstruation | 10 (10) |
| Food | 8 (8) |
| Fatigue | 1 (1) |
| Eye strain | 7 (7) |
| Hair wash | 23 (23) |
| Dehydration | 5 (5) |
| Exercise | 8 (8) |
| Duration of illness (mean in years) (SD, range in years) | 7.07 (5.40, 1–25) |
| Headache type at presentation | |
| Episodic headache | 51 (51) |
| Chronic daily headache | 49 (49) |
| Sleep character analysis, sleep abnormality present/no sleep abnormality (no. of patients, % of patients) | 57 (57)/43 (43) |
| Mean global PSQI score (SD, range) (A global PSQI score 5 or more considered as abnormal sleep) | 6.54 (3.407, 0–16) |
| Analysis of non-headache symptoms number of patients having symptoms (% of study population) | |
| Blurring of vision | 24 (24) |
| Diplopia | 9 (9) |
| Scotoma | 3 (3) |
| Periorbital pain | 59 (59) |
| Neck pain | 70 (70) |
| Nausea, vomiting | 69 (69) |
| Cervical muscle tenderness | 57 (57) |
| Conjunctival injection | 23 (23) |
| Nasal congestion | 15 (15) |
| Hemisensory pain | 19 (19) |
| Feeling tired | 73 (73) |
| Difficulty in concentration | 78 (78) |
Non-headache symptoms were analyzed depending on 12 commonly reported symptoms reported in earlier studies.[11] Among the non-headache symptoms, blurring of vision were present in 24 (24%), diplopia in 9 (9%), scotoma 3(3%), periorbital pain 59 (59%), neck pain 70 (70%), nausea and vomiting 69 (69%), cervical muscle tenderness 57 (57%), conjunctival injection 23 (23%), nasal congestion 15 (15%), hemisensory pain 19 (19%), feeling tired 73 (73%), and difficulty in concentration 78 (78%) were noted. Most frequently reported that non-headache symptoms were difficulty in concentration and tired feeling followed by neck pain, nausea, and vomiting [Table 1].
Demographic and clinical characters are compared between episodic (51 patients, 51%) and CM (49 patients, 49%) group. There was no significant difference in mean age 32.61 ± 10.35 versus 31.02 ± 8.88 years, P = 0.41. There was no significant difference in duration of illness in both the categories (7.18 ± 5.38 versus 6.95 ± 5.49 years, P = 0.83) between episodic and CM groups. Among the triggers, physical stress (P = 0.03), trigger by specific food (P = 0.03), and fatigue (P = 0.01) were significantly more prevalent in CM group [Table 2].
| Characteristics | Episodic (n=51) (%) | Chronic migraine group (n=49) (%) | P-value |
|---|---|---|---|
| Age (in years) (SD) | Mean 32.61 (10.35) | Mean 31.02 (8.88) | 0.413 |
| Duration of illness (years) (SD) | Mean 7.18 (5.37) | Mean 6.95 (5.49) | 0.835 |
| Gender | Female-36 (70.58) | Female-37 (75.51) | 0.579 |
| Male-15 (29.42) | Male-12 (24.49) | ||
| Comparison of triggers (frequency in percentage) | |||
| Fast | 12 (24) | 11 (22) | 0.098 |
| Hunger | 14 (27) | 15 (31) | 0.728 |
| Noise | 18 (35) | 31 (63) | 0.005 |
| Mental stress | 27 (53) | 26 (53) | 0.990 |
| Physical stress | 17 (33) | 27 (55) | 0.028 |
| Sun exposure | 42 (82) | 40 (82) | 0.925 |
| Cold | 9 (18) | 7 (14) | 0.647 |
| Hot | 11 (22) | 9 (18) | 0.689 |
| Weather change | 9 (18) | 13 (27) | 0.284 |
| Post-coital | 1 (2) | 1 (2) | 0.988 |
| Sleep deprivation | 20 (39) | 22 (45) | 0.565 |
| Perfume | 6 (12) | 10 (20) | 0.239 |
| Menstruation | 2 (5) | 8 (21) | 0.025 |
| Food | 3 (6) | 5 (10) | 0.426 |
| Exercise | 3 (6) | 5 (10) | 0.426 |
| Dehydration | 2 (5) | 3 (6) | 0.614 |
| Hair wash | 12 (25) | 11 (22) | 0.898 |
| Eye strain | 0 (0) | 7 (14) | 0.005 |
| Fatigue | 0 (0) | 1 (2) | 0.305 |
| Comparison of non-headache features (frequency in percentage) | |||
| Blurring of vision | 8 (16) | 16 (33) | 0.047 |
| Diplopia | 3 (6) | 6 (12) | 0.266 |
| Scotoma | 0 (0) | 3 (6) | 0.073 |
| Periorbital pain | 32 (63) | 27 (55) | 0.437 |
| Neck pain | 32 (63) | 38 (78) | 0.106 |
| Nausea, vomiting | 36 (71) | 33 (67) | 0.726 |
| Cervical muscle tenderness | 23 (45) | 34 (69) | 0.014 |
| Conjunctival injection | 12 (24) | 11 (22) | 0.898 |
| Nasal congestion | 3 (6) | 12 (24) | 0.009 |
| Hemisensory pain | 7 (14) | 12 (24) | 0.170 |
| Feeling tired | 36 (71) | 37 (76) | 0.579 |
| Difficult concentration | 39 (76) | 39 (80) | 0.706 |
| Allodynia | 11 (22) | 25 (51) | 0.002 |
| Comparison of sleep characters | |||
| Abnormal sleeper | 23 (45.1) | 34 (69.4%) | 0.014 |
| Global PSQI score (SD) | 5.16 (2.43) | 7.98 (3.69) | <0.001 |
| Epworth Sleepiness Scale score (SD) | 5.88 (4.51) | 6.41 (4.67) | 0.569 |
| Subjective sleep quality score | 1.12 (0.739) | 1.55 (0.843) | 0.008 |
| Sleep latency score | 1.12 (0.909) | 1.88 (1.103) | <0.001 |
| Sleep duration score | 0.96 (0.979) | 1.46 (0.874) | 0.009 |
| Sleep efficiency score | 0.49 (0.505) | 0.86 (0.913) | 0.016 |
| Sleep disturbance score | 0.90 (0.500) | 1.33 (0.718) | 0.001 |
| Use of sleep medicine score | 0.08 (0.337) | 0.27 (0.736) | 0.103 |
| Daytime dysfunction score | 0.51 (0.664) | 0.69 (0.713) | 0.179 |
Comparison of non-headache symptoms between the episodic and CM group revealed; blurring of vision (8 [16%] vs. 16 [33%], P = 0.05), nasal congestion (3 [6%] vs. 12 [24%], P = 0.01), and cervical muscle tenderness (23 [45%] vs. 34 [69%], P = 0.01) were more prevalent in chronic headache group. Allodynia was more prevalent in the CM group (P < 0.01). There was a significant difference in sleep quality in both the groups with global PQSI score which was significantly higher in the CM group (episodic vs. chronic 5.16 ± 2.43 vs. 7.98 ± 3.69, P < 0.01). PSQI questionnaire detected significantly higher score in all the points in chronic headache group. The PSQI 7 points between episodic and chronic migraineurs revealed: Subjective sleep quality score (1.12 ± 0.74 vs. 1.55 ± 0.84, P < 0.01), sleep latency score (1.12 ± 0.91 vs. 1.88 ± 1.10, P < 0.01), sleep duration score (0.96 ± 0.98 vs. 1.46 ± 0.87, P = 0.01), sleep efficiency score (0.49 ± 0.51 vs. 0.86 ± 0.91, P = 0.02), sleep disturbance score (0.90 ± 0.50 vs. 1.33 ± 0.72, P = 0.01), use of sleep medicine score (0.08 ± 0.34 vs. 0.27 ± 0.74, P = 0.10), and daytime dysfunction score (0.51 ± 0.66 vs. 69 ± 0.71, P = 0.18) [Table 2]. All the points detected significant difference in scores except in sleep medicine use and daytime dysfunction score. Epworth Sleepiness Scale score did not detect any significant difference in between groups and both the episodic and CM groups score was not suggestive of excessive day time somnolence (5.88 ± 4.51 vs. 6.41 ± 4.67, P = 0.57) [Figure 1].

- Bar diagram showing comparison of different characters in episodic and chronic migraine group.
Out of total 100 patients, 57 (57%) patients were identified as PSs (global PSQI >5) and rest 43 (43%) were GSs (global PSQI score ≤5). No significant difference was noted in demographic characters in both the groups including gender distribution (P = 01) and duration of illness (7.49 ± 5.33 vs. 6.50 ± 5.52 years, P = 0.90).
Significant difference was observed between poor versus GSs in; frequency of headache in the past 1 month (17.18 ± 10.16 vs. 11.53 ± 9.18 attacks per month, P = 0.01) and in total number of triggers (4.77 ± 2.52 vs. 3.79 ± 1.54, P = 0.02) [Table 3]. Among the triggers, physical stress (P = 0.04) was significantly more prevalent in PSs group [Table 3]. Comparison of non-headache symptoms and allodynia distribution between poor sleepers (PSs) and GSs group (GSs) did not revealed any significant difference between the two groups [Table 3].
| Characters | Poor sleepers (global PQSI >5) N 57 (57%) | Good sleepers (global PQSI ≤5) N 43 (43%) | P-value |
|---|---|---|---|
| Age in years (SD) | 32.61 (9.13) | 30.79 (9.958) | 0.935 |
| Duration of illness in years | 7.49 (5.326) | 6.50 (5.524) | 0.907 |
| Sex | |||
| Female | 45 (78.94) | 28 (65.11) | 0.123 |
| Male | 12 (21.06) | 15 (34.88) | |
| Total number of triggers, mean (SD) | 4.77 (2.515) | 3.79 (1.539) | 0.019 |
| Analysis of triggers | |||
| Fast | 15 (26.3) | 8 (18.6) | 0.364 |
| Hunger | 20 (35.1) | 9 (20.9) | 0.122 |
| Noise | 23 (40.4) | 26 (60.5) | 0.073 |
| Physical stress | 30 (52.6) | 14 (32.6) | 0.072 |
| Mental stress | 31 (54.4) | 22 (51.20) | 0.749 |
| Sun exposure | 50 (87.7) | 32 (74.4) | 0.087 |
| Cold | 10 (17.5) | 6 (14.0) | 0.628 |
| Hot | 12 (21.1) | 8 (18.6) | 0.762 |
| Weather change | 15 (26.3) | 7 (16.3) | 0.230 |
| Post-coital | 1 (1.8) | 1 (2.3) | 0.827 |
| Sleep deprivation | 23 (40.4) | 19 (44.2) | 0.700 |
| Perfume | 11 (19.3) | 5 (11.6) | 0.300 |
| Menstruation | 7 (12.3) | 3 (7.0) | 0.511 |
| Food | 6 (10.5) | 2 (4.7) | 0.284 |
| Analysis of non-headache symptoms | |||
| Blurring of vision | 15 (26.3) | 9 (20.9%) | 0.532 |
| Diplopia | 4 (7.0) | 5 (11.6%) | 0.425 |
| Scotoma | 2 (3.5) | 1 (2.3%) | 0.731 |
| Periorbital pain | 33 (57.9) | 26 (60.5%) | 0.796 |
| Neck pain | 44 (77.2) | 26 (60.5%) | 0.071 |
| Nausea | 43 (75.4) | 26 (60.5%) | 0.109 |
| Cervical tender | 13 (22.8) | 20 (46.5%) | 0.066 |
| Nasal congestion | 11 (19.3) | 4 (9.3%) | 0.166 |
| Conjunctival ingestion | 13 (22.8) | 10 (23.3%) | 0.958 |
| Hemisensory pain | 10 (17.5) | 9 (20.9%) | 0.669 |
| Feeling tired | 42 (73.7) | 31 (81.4%) | 0.895 |
| Conc. diff | 43 (75.4) | 35 (81.4%) | 0.477 |
| Allodynia | 23 (40.4) | 13 (30.2%) | 0.297 |
| Headache frequency analysis | |||
| Episodic | 23 (40.4) | 28 (65.1) | 0.014 |
| CDH | 34 (59.6) | 15 (34.9) | |
| Headache frequency last month in attacks mean (SD) | 17.18 (10.16) | 11.53 (9.17) | 0.005 |
To measure the strength or relationship between the factors to estimate the propensity of conversion from episodic to CM, Pearson correlation was performed between headache frequency, global PSQI score, duration of illness, total number of triggers, and total number of non-headache symptoms. Moderately significant “r” value noted in between headache frequency and global PQSI score and total number of triggers and total non-headache associated symptoms. Between headache frequency and global PQSI score showed significant positive correlation (r = 0.45, P < 0.01) [Figure 2]. Between total number of triggers and total non-headache associated symptoms revealed positive correlation (r = 0.44, P < 0.01).
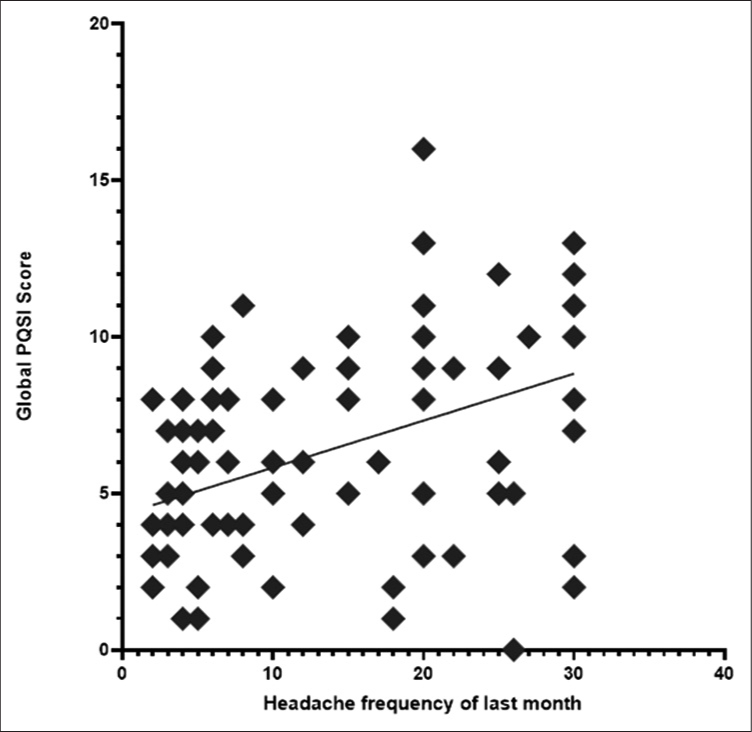
- Scatter plot showing that headache frequency and global PQSI score have significant positive correlation (r = 0.45, P < 0.01).
DISCUSSION
To the best of our knowledge, Indian data on migraine with attack-related variation depending on sleep quality are sparse. In our study, we found that headache frequency and global PQSI score have a moderately strong positive correlation. Among the well-known confounding factors, age, gender, duration of headache, character of headache, onset to peak time, and addiction were matched and patient having sleep-related breathing disorder, central disorder of hypersomnolence, Parasomnias, and sleep-related movement disorder[12] were excluded from our study.
There was significant difference in sleep quality in both the groups with global PQSI score which was significantly higher in the CM group. PSQI detected significantly higher score in all the points in chronic headache group except for the use of sleep medicine score and daytime dysfunction score. Epworth Sleepiness Scale score did not revealed significant difference between groups and also both the groups’ score was not suggestive of excessive daytime somnolence.
Premonitory symptoms occur up to 72 h before the onset of migraine pain. Such symptoms are likely hypothalamic in origin and include changes in sleep, arousal, mood, appetite, urination, and yawning. Maniyar et al.[13] first described abnormal activation of the hypothalamus during the premonitory phase, before the onset of head pain.[14,15] They found activations in the posterolateral hypothalamus, midbrain tegmental area, periaqueductal grey, dorsal pons, and various cortical areas including occipital, temporal, and prefrontal cortex. Brain activations, in particular of the hypothalamus, seen in the premonitory phase of glyceryl trinitrate-triggered migraine attacks as increased activity by positron emission tomography scans with H2O. Schulte et al. documented hypothalamic activation even 24 h before starting of head pain and additional to activation of trigeminocervical system and activity of mid-pons that establishes functionally active role of hypothalamus as migraine attack generator.[16] Role of anterior right hypothalamus in chronification of migraine is also established by Schulte et al.[16] Case reports and genetic data support the hypothesis of probable role of the HCRTR1 gene which may represent a genetic susceptibility for predisposition of migraine without aura and that the hypocretin neuronal system which is a key modulator of human sleep cycle may have a functionally active role in the pathophysiology of migraine.[15] Hence, from pathophysiological point, migraine attacks can have close relation with hypothalamic activity that ultimately determines attack frequency, chronicity, and hypersensitivity. Our study establishes significant frequent headache attacks and propensity to conversion to chronic headache in patients having worse subjective sleep quality score, sleep latency score, sleep duration score, sleep efficiency score, and sleep disturbance score in Indian scenario. This ultimately goes in favor of earlier study findings by Schulte et al. who depicted role of hypothalamus in chronicity of migraine.[16] On the other aspect, sleep and migraine may link in a bidirectional way and share some pathophysiological mechanisms through which sleep disturbances impacts on headache. The previous experimental studies have shown that sleep deprivation increases “self-reported pain.”[17] In addition, sleep deprived state may decrease the effect of pain modulating descending tracts along with that decrease in serotonin also shown to have role in modulation in pain inhibitory pathway that may lead to migraine generator pathophysiology modification. As chronicity is the strong predictor of disability, this aspect of migraine has immense therapeutic impact.
Neurophysiological studies have shown that the migraine brain is characterized by general neuronal hyperexcitability.[18] Evoked potentials and event-related potentials in migraine patients documented increased excitability to a wide range of stimuli including visual, somatosensory, and auditory, as well as brainstem reflexes in response to nociceptive stimuli.[18,19] This neuronal hyperexcitability which may be responsible for sensory sensitization which is underlying mechanism for allodynia also may be underlying pathophysiology behind this preservation of Epworth Sleepiness Scale score in spite of having poor sleeping habit.
In the literature, there are few studies documented sleep and primary headache relation. Song et al. reported that self-reported short sleep duration (<6 h) was associated with increased headache frequency in Korean population.[20] Sadeghniiat et al. reported increased PQSI score with poor sleep quality and more prevalent depressive symptoms in Iranian population.[21] Lucchesi et al. in a population-based study of Europe detected increased fatigue, BMI, poor sleep-wake pattern, and depressive and anxiety symptoms in CM patients rather than episodic group.[22] Kelman et al. in a study reported over half of the migraine patient reports difficulty in initiating sleep and maintaining sleep. Same study also reported that short sleep (<6 h) was associated with frequent headache and increased severity.[7] In an Indian study by Gupta et al., sleep disorder was shown to be more prevalent in primary headache group than normal population.[23] Lin et al. reported poor sleep quality related with high migraine frequency in both groups of migraine with aura and without aura group.[24] In a recent meta-analysis by Stanyer et al., sleep disturbance documented by polysomnography and subjective sleep quality, both were found to be more affected in migraine patients in comparison with normal population.[25] We found in our study that this sleep disturbance is more common in shift duty worker, lactating mothers where work patterns affect sleep pattern. We also noticed that smartphone overuse during bedtime also affecting attack rate which is easily modifiable by lifestyle modification though detail evaluation regarding this was not done but recent other study established similar finding.[26]
In our study, we also found that associated non-headache symptoms were more in CM group than episodic group. In our cohort, blurring of vision, scotoma, nasal congestion, and cervical muscle tenderness were more prevalent in chronic headache group along with allodynia. Functional imaging and PET imaging in migraine premonitory as well as ictal and postdrome phase have detected different cortical and subcortical areas of hyperactivity including posterolateral hypothalamus, anterior right hypothalamus, midbrain tegmental area, periaqueductal grey, dorsal pons, and various cortical areas including occipital, temporal, and prefrontal cortex which may explain the non-headache symptoms and “hyperexcitable brain” hypothesis may explain its more prevalence in chronic variety than episodic one along with significantly more allodynia in chronic group.
As a single-center study, sample size was not very high in our study and only questionnaire-based subjective sleep was evaluated. Although literature suggests subjective sleep correlates well with objective polysomnography, in primary headache disorder, the data are conflicting. In adult population, how this subjective sleep is being reflected in sleep architecture need further detailed study which can clearly guide therapeutic interventions. Associated mood disorder and anxiety disorders also have impact on sleep though these conditions were not evaluated in our study.
CONCLUSION
In our study, we found that headache frequency and global PQSI score have a moderately strong positive correlation suggestive of poor subjective sleep quality in migraine population with more frequent migraine attack. Chronic migraineurs have poor subjective sleep quality, increased sleep latency, decreased sleep duration, decreased sleep efficiency, and increased sleep disturbance. Non-headache symptoms and allodynia were more prevalent in PSs and in CM patients’ needs further evaluation for therapeutic consideration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Global burden of disease study 2017. Lancet. 2017;5:1-27. http://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf [Last accessed 2022 Sep 10]
- [Google Scholar]
- Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-9.
- [CrossRef] [PubMed] [Google Scholar]
- A phase-by-phase review of migraine pathophysiology. Headache. 2018;58:4-16.
- [CrossRef] [PubMed] [Google Scholar]
- The international classification of headache disorders In: Cephalalgia Vol 33. (3rd edition (beta version)). 2013. p. :629-808.
- [CrossRef] [PubMed] [Google Scholar]
- Proposed new diagnostic criteria for chronic migraine. Cephalalgia. 2020;40:399-406.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep and quality of life in the Austrian population. Acta Neurol Scand. 2000;102:249-57.
- [CrossRef] [PubMed] [Google Scholar]
- Headache and sleep: Examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904-10.
- [CrossRef] [PubMed] [Google Scholar]
- Morning headaches in patients with sleep disorders. Sleep Med. 2003;4:377.
- [CrossRef] [PubMed] [Google Scholar]
- Migraine and sleep disorders: A systematic review. J Headache Pain. 2020;21:126.
- [CrossRef] [PubMed] [Google Scholar]
- The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic syndrome and insulin resistance in migraine. J Headache Pain. 2012;13:321-6.
- [CrossRef] [PubMed] [Google Scholar]
- International classification of sleep disorders-third edition: Highlights and modifications. Chest. 2014;146:1387-94.
- [CrossRef] [PubMed] [Google Scholar]
- Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137:232-41.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted orexin and hypothalamic neuropeptides for migraine. Neurotherapeutics. 2018;15:377-90.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for an association between migraine and the hypocretin receptor 1 gene. J Headache Pain. 2011;12:193-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothalamus as a mediator of chronic migraine: Evidence from high-resolution fMRI. Neurology. 2017;88:2011-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357-69.
- [CrossRef] [PubMed] [Google Scholar]
- Highlights in migraine electrophysiology: Are controversies just reflecting disease heterogeneity? Curr Opin Neurol. 2016;29:320-30.
- [CrossRef] [PubMed] [Google Scholar]
- The electrophysiology of migraine. Curr Opin Neurol. 2003;16:327-31.
- [CrossRef] [PubMed] [Google Scholar]
- Short sleep duration and poor sleep quality among migraineurs: A population-based study. Cephalalgia. 2018;38:855-64.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep quality and depression among patients with migraine. Acta Med Iran. 2013;51:784-8.
- [Google Scholar]
- Fatigue, sleep-wake pattern, depressive and anxiety symptoms and body-mass index: Analysis in a sample of episodic and chronic migraine patients. Neurol Sci. 2016;37:987-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of primary headaches on subjective sleep parameters among adolescents. Ann Indian Acad Neurol. 2008;11:164-9.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between sleep quality and migraine frequency. Medicine (Baltimore). 2016;95:e3554.
- [CrossRef] [PubMed] [Google Scholar]
- Subjective sleep quality and sleep architecture in patients with migraine: A meta-analysis. Neurology. 2021;97:e1620-31.
- [CrossRef] [PubMed] [Google Scholar]
- Smartphone use and primary headache: A cross-sectional hospital-based study. Neurol Clin Pract. 2020;10:473-9.
- [CrossRef] [PubMed] [Google Scholar]






