Translate this page into:
Surgical treatment of epilepsy - Initial experience from a comprehensive epilepsy program in coastal South India
*Corresponding author: Girish Menon, Department of Neurosurgery, Kasturba Medical College, Manipal Academy of Higher Education, Udupi, Karnataka, India. girish.menon@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Shenoy N, Srinivasan S, Menon G, Kurupath R. Surgical treatment of epilepsy - Initial experience from a comprehensive epilepsy program in coastal South India. J Neurosci Rural Pract 2023;14:488-94.
Abstract
Objectives:
The objectives of this study were to share our initial experience with epilepsy surgery and provide an overview on the surgical treatments of epilepsies.
Materials and Methods:
This was a retrospective analysis of the demographics and clinical and investigative features of patients who underwent epilepsy surgery between January 2016 and August 2021. Postoperative seizure outcome was categorized according to modified Engel’s classification, and the minimum period of follow-up was 1 year.
Results:
The study group included 30 patients with an age ranging from 6 years to 45 years (mean: 22.28 years, median: 20 years) and a male: female ratio of 20:10. The epilepsy duration before surgery ranged from 3 years to 32 years (median: 7 years). Majority of our patients underwent resective surgeries (28/30 = 93.3%), and disconnection procedures were done in two patients. This included one functional hemispherotomy and one posterior quadrantic disconnection. Temporal lobe resective surgery was the most common procedure (16/30 = 53.3%), followed by eight frontal lobe and two parietal lobe surgeries. Among resective surgeries, majority were lesional surgeries and the pathologies included mesial temporal sclerosis (4), dysembryoplastic neuroepithelial tumor (8), ganglioglioma (6), cavernoma (4), focal cortical dysplasia (2), gliosis (1), and one case of hypothalamic hamartoma. Intraoperative electrocorticography was used in all cases for optimizing surgical resection/disconnection. Nearly two-thirds of our patients (66.6%) had an Engel’s Class I outcome, five patients had Engel’s Class II outcome, three patients had Class III outcome, and one patient did not have any worthwhile improvement. Temporal lobe surgery patients had a better seizure outcome compared to extratemporal surgeries (84% vs. 74%). Overall, complications were minimal and short lasting, and comprised meningitis in three patients (5.6%) and transient worsening of hemiparesis following hemispherotomy in two patients. There was no mortality or long-lasting major morbidity in our patients.
Conclusion:
In carefully selected patients with drug-resistant epilepsy, surgery offers an excellent chance of becoming seizure-free with significant improvement in overall quality of life. Majority of the common epilepsy surgery procedures can be performed through a multidisciplinary approach even in centers with limited resources.
Keywords
Drug-resistant epilepsy
Antiseizure medication
Surgery for epilepsy
Electrocorticography
Temporal lobectomy
Hemispherotomy
INTRODUCTION
Surgical treatment has become a viable and effective option for patients with drug-resistant epilepsy (DRE) who are poorly responsive to antiseizure medications (ASMs).[1-3] Given that about 20% of individuals with epilepsy are resistant to ASMs, there would be approximately one million potential candidates for epilepsy surgery in India.[3,4] Yet, only <1000 epilepsy surgeries are currently being done annually and only 2 out of every 1000 eligible patients undergo epilepsy surgery in India.[4,5] This article attempts to share the initial successful experience with surgery for refractory epilepsy in a tertiary care center in coastal South India and attempts to highlight the beneficial effects of this underutilized surgical option for epilepsy.
MATERIALS AND METHODS
This was a retrospective analysis of case records of all patients who underwent epilepsy surgery between January 2016 and August 2021. We defined epilepsy surgery as those primarily undertaken for uncontrolled epileptic seizures, and excluded patients with epilepsy in whom the surgery was done per se for a lesion. We used the International League Against Epilepsy consensus statement which defines drug resistance as the failure of two appropriate ASMs administered in adequate doses to achieve sustained seizure freedom.[6] Presurgical evaluation involved a detailed history of the antecedents, onset, and semiology of the epileptic seizures, neurological examination, 1.5 or 3 T magnetic resonance imaging (MRI) according to epilepsy protocol, prolonged video-electroencephalogram (EEG) monitoring, and neuropsychological assessment. The presurgical evaluation data were thoroughly discussed in the multidisciplinary patient management conference comprising a team of neurologist, neurosurgeon, radiologist, psychologist, and EEG technologist before selecting for epilepsy surgery. Patients were followed up after surgery at 3 months, 1 year, and thereafter annually or more frequently, if necessary. We categorized the postoperative seizure outcome according to modified Engel’s classification.[2]
RESULTS
Our study group [Table 1] included 30 patients with an age ranging from 6 years to 45 years (mean: 22.28 years, median: 20 years) and a male: female ratio of 20:10. The epilepsy duration before surgery ranged from 3 years to 32 years (median: 7 years). Majority of our patients underwent resective surgeries (28/30 = 93.3%), and disconnection procedures were done in only two patients. The first patient was a 7-year-old boy who had evidence of refractory complex partial seizure with ictal localization in the left parietal and posterior temporo-occipital region. He underwent a posterior temporo-parieto-occipital disconnection surgery. The second patient was another 7-year-old boy who presented with refractory epilepsy of the left hemispheric origin secondary to significant gliosis, encephalomalacic changes, and cortical atrophy. He underwent a vertical paramedian parasagittal hemispherotomy. Temporal lobe resective surgery was the most common procedure (16/30 = 53.3%), followed by eight frontal lobe and two parietal lobe surgeries. Among resective surgeries, majority were lesional surgeries and the pathologies included mesial temporal sclerosis (4), dysembryoplastic neuroepithelial tumor (DNET) (8), ganglioglioma (6), cavernoma (4), focal cortical dysplasia (2), gliosis (1), and one case of hypothalamic hamartoma. Intraoperative electrocorticography was used in all cases for optimizing surgical resection/disconnection. Except for one patient who needed bilateral craniotomy for surface grid electrode and hemispheric localization of epileptogenic focus, none of the other patients underwent any form of invasive preoperative monitoring.
| Patient no. | Age | Diagnosis | Pathology | Surgery | In this column add outcome |
|---|---|---|---|---|---|
| 1 | 36/M | Right posterior frontal epilepsy | Cortical dysplasia | Lesionectomy | II |
| 2 | 26/F | Right mesial temporal lobe epilepsy | MTS | ATL with AH | I |
| 3 | 14/M | Left temporal lobe epilepsy | DNET | ATL, AH, and lesionectomy | I |
| 4 | 28/M | Right temporal lobe epilepsy | DNET | Right ATL+AH lesionectomy | I |
| 5 | 7/M | Left hemispheric epilepsy | Gliosis | Vertical parasagittal left hemispherotomy | II |
| 6 | 7/M | Left posterior cortex epilepsy | Gliosis | Left temporal lobectomy with posterior quadrantectomy - temporo-parieto-occipital disconnection | II |
| 7 | 20/F | Right mesial temporal lobe epilepsy | MTS | ATL with AH | I |
| 8 | 21/M | Right mesial temporal sclerosis | MTS | ATL with AH | I |
| 9 | 40/F | Right mesial temporal lobe epilepsy sclerosis | MTS | ATL with AH | I |
| 10 | 6/F | Right frontal lobe epilepsy | Cortical dysplasia | Lesionectomy | II |
| 11 | 16/M | Left frontal lobe epilepsy | Gliosis | Complete excision of lesion under cortical electrophysiological monitoring | II |
| 12 | 27/M | Left frontal lobe epilepsy | Ganglioglioma | Awake craniotomy and excision of ganglioglioma | III |
| 13 | 49/M | Right frontal lobe epilepsy | Cavernoma | Lesionectomy | I |
| 14 | 24/M | Right temporal lobe epilepsy | Ganglioglioma | ATL with AH with lesionectomy | I |
| 15 | 7/M | Right temporal lobe epilepsy | Ganglioglioma | ATL with AH with lesionectomy | I |
| 16 | 7/F | Right temporal lobe epilepsy | DNET | ATL with AH with lesionectomy | I |
| 17 | 4/M | Right temporal lobe epilepsy | Ganglioglioma | ATL with AH with lesionectomy | I |
| 18 | 14/F | Right temporal Lobe epilepsy | Low-grade glioma | ATL with lesionectomy | I |
| 19 | 14/M | Right temporal lobe epilepsy | DNET | ATL with lesionectomy | I |
| 20 | 28/M | Right temporal lobe epilepsy | DNET | ATL with lesionectomy | I |
| 21 | 46/M | Right frontal lobe epilepsy | Cavernoma | Lesionectomy under ECOG | I |
| 22 | 25/F | Right temporal lobe epilepsy | Ganglioglioma | ATL with lesionectomy | I |
| 23 | 27/M | Left temporal lobe epilepsy | Cavernoma | Lesionectomy | I |
| 24 | 29/F | Right frontal lobe epilepsy | Ganglioglioma | Lesionectomy | III |
| 25 | 9/M | Left parietal lobe epilepsy | Ganglioglioma | Lesionectomy | IV |
| 26 | 20/F | Left frontal lobe epilepsy | DNET | Lesionectomy | I |
| 27 | 38/F | Right temporal lobe epilepsy | DNET | ATL with AH with lesionectomy | I |
| 28 | 11/M | Right parietal lobe epilepsy | DNET | Lesionectomy | III |
| 29 | 45/M | Right temporal lobe epilepsy | Cavernoma | Lesionectomy | I |
| 30 | 12/M | Hypothalamic gelastic epilepsy | Hamartoma | Hamartoma resection | II |
DNET: Dysembryoplastic neuroepithelial tumor, ECOG: Electrocorticography, ATL with AH: Anterior temporal lobectomy with amygdalohippocampectomy, MTS: Mesial temporal sclerosis
All the 30 patients had more than 1 year of postoperative follow. The seizure outcome of our series of 30 patients is shown in [Table 2]. Nearly two-thirds of our patients (66.6%) had an Engel’s Class I outcome, five patients had Engel’s Class II outcome, while three patients had Class III outcome and one patient did not have any worthwhile improvement. One patient with a hypothalamic hamartoma had to be reoperated due to persistent gelastic seizures and a residual lesion. His seizures are now well controlled following the second surgery. Temporal lobe surgery patients had a better seizure outcome compared to extratemporal surgeries (84% vs. 74%). Both the patients who underwent disconnection procedures had Engel’s Grade II outcome. Overall, complications were minimal and short lasting, and comprised meningitis in three patients (5.6%) and transient worsening of hemiparesis following hemispherotomy in two patients. There was no mortality or long-lasting major morbidity in our patients.
| Class I | Class II | Class III | Class IV | |
|---|---|---|---|---|
| Lesion wise | ||||
| MTS (04) | 04 | |||
| DNET (08) | 07 | 01 | ||
| Ganglioglioma (7) | 04 | 02 | 01 | |
| Cavernoma (4) | 04 | |||
| Gliosis (03) | 03 | |||
| Cortical dysplasia (2) | 02 | |||
| Hypothalamic hamartoma | 01 | |||
| Type of surgery | ||||
| Resection (28) | 20 | 04 | 03 | 01 |
| Disconnection (02) | 02 | |||
| Site of surgery | ||||
| Temporal (18) | 17 | 01 | ||
| Extratemporal (11) | 3 | 4 | 3 | 1 |
| Hypothalamus (01) | 01 | |||
DNET: Dysembryoplastic neuroepithelial tumor
DISCUSSION
The majority of epileptic patients who will eventually achieve adequate seizure control typically do so within 2 years of the onset of epilepsy, while the remainder are more likely to develop DRE.[3,7] Recurrent seizures can result in severe psychosocial, familial, educational, financial, and occupational consequences. Surgery in selected patients with DRE helps to achieve successful reduction in seizures and may enable ASM withdrawal in some. Surgery also helps in psychosocial, educational, occupational and overall improvement in quality of life.[2,3,8]
When should epilepsy surgery be considered?
Delaying surgery until a patient’s lifestyle has become established could result in the surgery having little impact on the individual’s quality of life. Therefore, surgery should be considered early in suitable patients to effectively eliminate seizures.[2,3,8-10] In addition, in certain surgically remediable epilepsy syndromes, surgery needs to be considered early even without waiting unduly to establish drug resistance. We have adopted a policy of early surgery in appropriate patients, and accordingly, the mean age in our series was 22 years and the median epilepsy duration before surgery was 7 years.
Defining refractoriness to medications
We follow a simple “rule of two” to define refractoriness – “If after 2 years of appropriate ASMs (closely monitored trials of ASM including two separate trials with a single medication and one trial with a combination of two medications), the patient continues to have more than two seizures every month, we recruit the patient for consideration for surgery.[6,11] Before categorizing a patient as having DRE, pseudo-refractoriness due to suboptimal dosage of ASMs, diagnostic errors, inappropriate choice of ASMs, poor compliance to drugs, and unhealthy lifestyles (such as erratic sleep, overindulgence in alcohol and recreational drugs) should be ruled out.[6,11,12]
Surgically remediable epilepsy syndromes
Surgically remediable epilepsy syndromes refer to epilepsy associated with cortical dysplasia, mesial temporal sclerosis, benign tumors (eg: ganglioglioma, Dysembryoplastic neuroepithelial tumour (DNET)), vascular malformations, and specific paediatric epilepsy conditions such as hemimegaloencephaly and Rasmussen’s encephalitis [Figure 1].[11,13] As has been described in the literature, the most prevalent surgically treatable lesional adult epilepsy syndrome in our series was mesial temporal lobe epilepsy secondary to mesial temporal lobe sclerosis. However, in childhood epilepsy cases, focal cortical dysplasia is more dominant.[3,8,13]
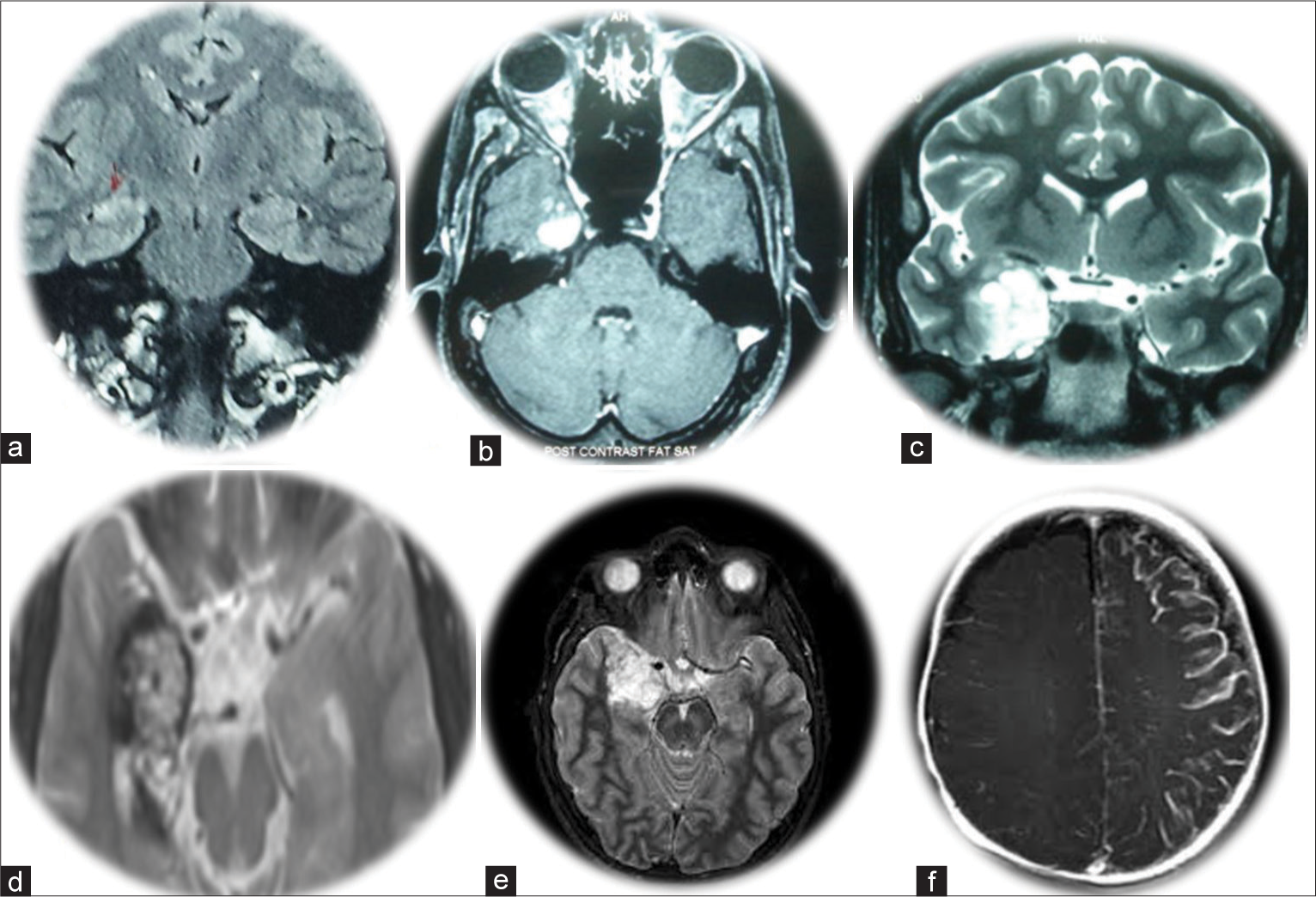
- Magnetic resonance imaging images of selected patients with surgically remediable epilepsy syndromes. (a) Right temporal mesial temporal sclerosis (Patient 8); (b) Right temporal gangliogliomas (Patient 14); (c) Right temporal uncal low-grade glioma (Patient 14); (d) Right temporal cavernoma (Patient 29); (e) Right temporal dysembryoplastic neuroepithelial tumor (Patient 20); (f) Rasmussen’s encephalitis (Patient 5).
Contraindications for surgery
Epilepsy surgery is contraindicated in primary generalized epilepsy, minor seizures that do not significantly impact the patient’s quality of life, progressive medical or neurological disorders, active psychosis, behavioral problems that interfere with rehabilitation, and poor contralateral memory function.[11,13]
Presurgical evaluation
Presurgical evaluation requires a multidisciplinary approach comprising neurologists, neurosurgeons, radiologists, psychologists, psychiatrists, and medico-social workers.[6,13-15] The objective of the presurgical evaluation is (a) to confirm the diagnosis of epileptic seizures while ruling out nonepileptic behavioral events, (b) identify the lesion causing the seizures, (c) choose suitable surgical candidates based on an optimal combination of electro-clinical-radiological correlation, and (d) ensure that surgery will not lead to significant neuropsychological impairments.[6,13] The key components of the presurgical evaluation include a thorough clinical history and physical examination, video-EEG monitoring, high-resolution MRI, neuropsychological testing, and evaluation of psychosocial well-being. The second stage may involve functional studies such as SPECT and PET, advanced modalities like magnetic source imaging (MSI), EEG-functional MRI (EEG-fMRI), and electrical source imaging (ESI). Invasive intracranial monitoring is reserved as the last resort. Wada test and functional MRI for language and memory functions, cortical stimulation, and mapping during intracranial monitoring help in predicting and minimizing deficits.[6,13-15] In recent years, stereotactically placed intracranial electrodes and EEG recording (stereo-EEG) have made invasive monitoring less traumatic and more accurate.[16] However, presurgical evaluation needs to be customized and optimized and costly advanced and invasive monitoring should be judiciously reserved for few complex cases. A good majority of epilepsy surgery work can be performed with basic investigations as was observed in our series where we performed invasive monitoring in only one patient.
Surgical treatment
The goal of epilepsy surgery is the complete removal/ disconnection of the “epileptogenic zone,” which is defined as the area necessary and sufficient for initiating seizures, and resection/disconnection which is associated with sustained seizure freedom.[2,6,13] Surgical techniques can be of three types - resective, disconnection, or augmentative.
Resective surgeries
The most frequent surgical approach used to treat epilepsy in adults is anterior temporal lobectomy in combination with amygdalohippocampectomy.[3,8,17,18] The “standard” anterior temporal lobectomy surgery involves the removal of a 5–6 cm section of temporal neocortex, including the superior temporal gyrus, the anterior two-thirds of the hippocampus, the lateral two-thirds of the amygdala, as well as the uncus and parahippocampal gyrus.[3,17,18] In selected patients, neocortical sparing selective amygdalohippocampectomy provides comparable results.[19,20] More than two-thirds of the resected specimens show hippocampal neuronal loss with sclerosis (mesial temporal sclerosis), which is the most common pathology observed.[17,19,20] Other examples of resective epilepsy surgery involve tailored resection (under intraoperative electrophysiological monitoring) of an “epileptogenic zone” in any lobe of the brain. The culprit zone may harbor an underlying pathological substrate (gliotic brain, cortical dysplasia, cavernoma, hamartoma, DNET, ganglioglioma, etc.). Extratemporal resections account for only 10–20% of epilepsy surgeries but is more challenging because it is often difficult to define the epileptogenic zone.[21,22] Stereotactic lesionectomy and volumetric resection of seizure focus help to reduce morbidity, especially in deep-seated and eloquent cortex lesions.[18] Hemispherectomy, a form of resective surgery earlier undertaken in patients with large hemispheric lesions, has now been completely replaced by functional hemispherotomies.
Disconnection procedures
In patients who have multiple epileptic foci, multilobar resections may be necessary, which can result in significant complications. An alternative approach is to perform a disconnection surgery which allows the functional isolation of the hemisphere or multiple lobes.[23-29] The commonly performed disconnection surgeries include hemispherotomy, quadrantic disconnections, corpus callosotomy, and multiple subpial disconnections.
Functional hemispherotomy is the most commonly performed disconnection surgery where the epileptogenic hemisphere is functionally isolated. The common indications for functional hemispherotomy are children with refractory hemiplegic epilepsy which include a number of disorders such as congenital neuronal migrational defects (cortical dysplasia, hemimegalencephaly, and hemiconvulsion–hemiplegia–epilepsy syndrome) and certain destructive lesions of a unilateral hemisphere (congenital porencephaly, perinatal cerebrovascular accidents, Sturge–Weber syndrome, and Rasmussen’s encephalitis). The surgical approach has evolved over the years, and the widely used techniques fall into two basic categories – lateral and vertical approaches. The lateral approach uses a corridor around the Sylvian fissure (peri-insular functional hemispherotomy advocated by Villemure and Mascott[26]), and the vertical approach proceeds down from the vertex (transcortical transventricular approach popularized by Delalande et al.[25]). Either of these techniques essentially involves four major steps: (1) disconnection of the corticothalamic tract (internal disconnection of the internal capsule and corona radiata); (2) resection of the medial temporal structures; (3) total corpus callosotomy; and (4) and disconnection of the orbito-fronto-hypothalamic tract. In “peri-insular hemispherotomy,” this goal is achieved through three surgical stages: supra-insular window, infra-insular window, and insula resection. “Vertical parasagittal hemispherotomy” achieves the same type of disconnection but through a posterior frontal transcortical transventricular window. The technique of endoscopic hemispherotomy is the latest advancement, pioneered by Dodammani et al.[29] Majority of patients requiring hemispherotomy are children where minimal access endoscopic surgeries are advantageous in terms of minimizing blood loss and morbidity compared with the open methods.
Quadrantic disconnection is indicated when the epileptogenic zone encompasses large areas of the frontal and parietal lobes (anterior quadrant), or the temporal, parietal, and occipital lobes (posterior quadrant) in one hemisphere.[24] Quadrantic disconnection surgeries are considered when hemispherotomy is contraindicated in the presence of residual voluntary motor function of the contralateral limbs. Posterior quadrantic disconnection surgery is the most commonly performed quadrantic disconnection surgery and is done when the epileptogenic zone is localized to the posterior temporal, parietal, and occipital lobes. Gliosis due to perinatal insult, cortical dysplasias, and Sturge–Weber syndrome are the common indications. The surgical steps include (a) lateral (temporal) disconnection, followed by (b) mesial disconnection which involves resection of the head of the hippocampus and the ventral amygdala, (c) parietal cortical disconnection which involves disconnecting the cortex (behind the post-central gyrus and sensory cortex) from superior sagittal sinus, superiorly to the splenium inferiorly, (d) intraventricular sectioning of the splenium along the junction of the superior and medial wall of the atrium, and (e) sectioning of the fornix.[24]
Disconnection, endoscopic or through laser ablation, is being increasingly adopted for gelastic seizures secondary to small hypothalamic hamartomas with good results.[30]
Corpus callosotomy is a surgical procedure that is used as a palliative treatment for bihemispherical multifocal epilepsy, such as in Lennox–Gastaut syndrome, which results in frequent generalized seizures, especially those associated with drop attacks and injuries.[31]
Multiple subpial transection is a technique used when the epileptogenic focus is in an eloquent unresectable cortex. The technique involves making cuts perpendicular to the gyrus, from one sulcus to the other, and repeated roughly every 5 mm. This severs intracortical fibers along the disconnection but spares subcortical white matter and U-fibers, thereby preventing ictal spread, and reportedly provides a 55% benefit in seizure outcomes.[32]
Augmentative procedures
Vagus nerve stimulation is a treatment option for patients with non-lesional epilepsy, patients with multiple lesions which are not surgically removable, patients lacking clear cut lateralizing evidence, and for patients with cryptogenic generalized epilepsy that is unresponsive to ASM treatment. Deep brain stimulation is another augmentative procedure reserved for very selected patients in whom resective surgeries cannot be performed. Radiosurgery and laser ablation for mesial temporal sclerosis and hypothalamic hamartomas are newer minimally invasive surgical strategies, the long-term outcome of which is at present uncertain.[33]
Complications of epilepsy surgery
The rate of complications of epilepsy surgery has significantly reduced over the years to <3% risk of morbidity.[8,24-33] Complications that can arise from anterior temporal lobectomy include homonymous superior quadrantanopia, which can result from the involvement of the optic tract or radiation, as well as language deficits and manipulation hemiplegia caused by vascular injury or spasm affecting the Sylvian vessels and anterior choroidal artery branches. Frontal lobe resections may cause personality changes, while resection of the dominant inferior parietal lobule may lead to Gerstmann syndrome. Functional hemispherotomy has minimised risk of hydrocephalus and superficial cerebral hemosiderosis, the two most dreaded complications seen following hemispherectomy. Transient aggravation of hemiparesis following hemispherotomy is common, especially for hand functions, but permanent deficits such as hemiplegia are uncommon. Risk of infracts, brain swelling, and perioperative mortality is <1%, but visual field defects remain an expected complication following hemispheric and quadrantic disconnection. Callosotomy can result in the syndrome of mutism, apraxia of the non-dominant leg, and incontinence, although these are often transient.[31,33]
Outcome
Multiple trials have provided conclusive evidence that surgery is more effective than prolonged medical therapy in patients with refractory temporal lobe epilepsy where long-term seizure freedom can be achieved in about 70% (62–83%) of cases with low morbidity and mortality (<1%).[1,8,18,19,33,34] With extratemporal resective surgeries, the outcome is generally 10% less than temporal lobe surgeries. In hemispherotomies, the probability of achieving 1-year remission is 57% by 1 year, 70% by 2 years, and 77% by 7 years.[25-29] Large series on quadrantic disconnection surgeries are limited, but available reports suggest that temporo-parieto-occipital disconnection surgeries provide a good outcome in nearly 75% of patients.[24]
Prolonged longitudinal follow-up is essential for accurate assessment of seizure outcome, since initially seizure-free patients may relapse, and some patients with early postoperative seizure may become seizure-free subsequently (running-down phenomenon).[33,34] The proportion of seizure-free adults in whom ASMs have been withdrawn varied widely across studies, from 19% to 63% after around 5 years of seizure freedom. Nearly two-thirds of our patients (66.6%) had an Engel’s Class I outcome, five patients had Engel’s Class II outcome, while three patients had Class III outcome and one patient did not have any worthwhile improvement. Suboptimal outcome in three patients with ganglioglioma (Class III on two and Class IV in one) is probably related to incomplete excision as the lesions were near eloquent areas. Similarly, the two patients with cortical dysplasia had Class II outcome probably related to residual epileptogenic zone in the tail of the dysplasia. However, it needs to be emphasized that treatment outcome in epilepsy is not completely about seizure freedom and ASM withdrawal. Even a moderate reduction in seizure frequency can result in psychosocial, educational, and occupational and overall improvement of the quality of life.[33,34]
Determinants of postoperative seizure outcome
Few predictors of good outcomes after epilepsy surgery include younger age at surgery, shorter duration of epilepsy, absence of a history of status epilepticus, presence of a well-defined radiological lesion, concordance between clinical, EEG, and imaging data, absence of intellectual impairment, favorable psychosocial and occupational conditions, and the absence of comorbidities.[8,18-20,33,34] In patients undergoing hemispherotomy, favorable predictors include acquired etiologies such as Rasmussen’s encephalitis, porencephaly secondary to perinatal stroke, and Sturge–Weber syndrome. Cortical malformations such as hemimegalencephaly, cortical dysplasia, contralateral hemispheric pathology, and a long duration of epilepsy before surgery imply poor outcome following hemispherotomy.[25-29]
CONCLUSION
In carefully selected patients with DRE, surgery offers an excellent chance of becoming seizure-free with significant improvement in overall quality of life. Most of the epilepsy surgery procedures can be successfully performed through a multidisciplinary approach even in centers with limited resources.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-8.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in the management of epilepsy in resource-poor countries. Nat Rev Neurol. 2009;5:323-30.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment of low cost epilepsy surgery centres in resource poor setting. Seizure. 2019;69:245-50.
- [CrossRef] [PubMed] [Google Scholar]
- Concept of epilepsy surgery and presurgical evaluation. Epileptic Disord. 2015;17:19-31.
- [CrossRef] [PubMed] [Google Scholar]
- Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-77.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis. Brain. 2005;128:1188-98.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology as a risk factor for medically refractory epilepsy: A case for early surgical intervention. Neurology. 1998;5:1243-4.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome after seizure recurrence on antiepileptic drug withdrawal following temporal lobectomy. Neurology. 2018;91:e208-16.
- [CrossRef] [PubMed] [Google Scholar]
- Practice parameter: Temporal lobe and localized neocortical resections for epilepsy. Neurology. 2003;60:538-47.
- [CrossRef] [PubMed] [Google Scholar]
- An estimate of the prevalence of psychogenic non-epileptic seizures. Seizure. 2000;9:280-1.
- [CrossRef] [PubMed] [Google Scholar]
- Multimodal approaches in the evaluation of patients for epilepsy surgery. Clin Neurophysiol. 1999;50:40-52.
- [Google Scholar]
- The role of scalp EEG in the presurgical evaluation of patients with medically refractory temporal lobe epilepsy. Am J Electroneurodiagn Technol. 2001;41:116-35.
- [CrossRef] [Google Scholar]
- Noninvasive investigations successfully select patients for temporal lobe surgery. J Neurol Neurosurg Psychiatry. 1997;63:327-33.
- [CrossRef] [PubMed] [Google Scholar]
- Stereoelectroencephalography: Indication and efficacy. Neurol Med Chir (Tokyo). 2017;15:375-85.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment for mesial temporal lobe epilepsy associated with hippocampal sclerosis. Rev Neurol (Paris). 2015;171:315-25.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosurgical approaches to pediatric epilepsy: Indications, techniques, and outcomes of common surgical procedures. Seizure. 2020;77:76-85.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: A multivariate study. Neurology. 1998;51:465-71.
- [CrossRef] [PubMed] [Google Scholar]
- Seizure outcome after anterior temporal lobectomy and its predictors in patients with apparent temporal lobe epilepsy and normal MRI. Epilepsia. 2004;45:803-8.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical outcome in extratemporal epilepsies based on multimodal pre-surgical evaluation and sequential intraoperative electrocorticography. Behav Sci (Basel). 2021;11:30.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment for extratemporal epilepsy. Curr Treat Options Neurol. 2004;6:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- From resection to disconnection for seizure control in pediatric epilepsy children. J Korean Neurosurg Soc. 2019;62:336-43.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior quadrantic epilepsy surgery: Technical variants, surgical anatomy, and case series. Epilepsia. 2007;48:1429-37.
- [CrossRef] [PubMed] [Google Scholar]
- Vertical parasagittal hemispherotomy: Surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60:ONS19-32.
- [CrossRef] [PubMed] [Google Scholar]
- Peri-insular hemispherotomy: Surgical principles and anatomy. Neurosurgery. 1995;37:975-81.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric functional hemispherectomy: Outcome in 92 patients. Acta Neurochir (Wien). 2012;154:2017-28.
- [CrossRef] [PubMed] [Google Scholar]
- The Treatment of Epilepsy: Principles and Practice (2nd ed). Baltimore: Williams and Wilkins; 1997. p. :1074-80.
- [Google Scholar]
- Endoscopic hemispherotomy for nonatrophic rasmussen's encephalopathy. Neurol India. 2021;69:837-41.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic treatment of hypothalamic hamartomas. J Korean Neurosurg Soc. 2017;60:294-300.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical aspects of corpus callosotomy. Brain Sci. 2021;11:1608.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple subpial transections for medically refractory epilepsy: A disaggregated review of patient-level data. Neurosurgery. 2018;82:613-20.
- [CrossRef] [PubMed] [Google Scholar]
- Seizure outcomes in nonresective epilepsy surgery: An update. Neurosurg Rev. 2017;40:181-94.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes of surgical treatment for epilepsy in adults with regard to seizures, antiepileptic drug treatment and employment. Seizure. 2017;44:217-24.
- [CrossRef] [PubMed] [Google Scholar]






