Translate this page into:
Intraoperative Measurement of Intracranial Pressure During Cranial Vault Remodeling in Children with Craniosynostosis
Dhaval Shukla, MCh Department of Neurosurgery, National Institute of Mental Health and Neurosciences Bengaluru, Karnataka 560029 India neurodhaval@rediffmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Purpose In this study, we analyzed the utility of intracranial pressure (ICP) monitoring intraoperatively for deciding height reduction and need for cerebrospinal fluid (CSF) diversion during cranial vault remodeling in children with multisutural craniosynostosis (CS).
Methods This is a retrospective observational study of children who underwent surgery for CS and ICP monitoring during surgery. The ICP was monitored using an external ventricular drainage catheter. The ICP monitoring was continued during the entire procedure.
Results A total of 28 (19 boys) children with the involvement of two or more sutures underwent ICP monitoring during surgery. The commonest pattern of suture involvement was bicoronal seen in 16 (57.1%) children followed by pancraniosynostoses in eight (28.6%) cases. The mean opening ICP was 23 mm Hg, which dropped to 10.9 mm Hg after craniotomy. The ICP increased transiently to 19.5 mm Hg after height reduction, and the mean ICP at closure was 16.2 mm Hg. The ICP recordings helped in undoing the height reduction in two children and ventriculoperitoneal shunt after surgery in two children.
Conclusions Intraoperative monitoring of ICP helps in deciding the type of cranial vault remodeling and the need for CSF diversion after surgery.
Keywords
Intracranial pressure
craniosynostosis
syndromic CS
VP shunt
cranial vault remodeling
Introduction
Craniosynostosis (CS) results in increased intracranial pressure (ICP), the incidence being higher (approximately 30–40%) in multi-sutural or syndromic variety of CS.1 The non-syndromic patients are also at the risk of having intracranial hypertension, and this is related to the number of sutures involved. The pathophysiology of intracranial hypertension is multifactorial, the predominant etiology being the premature fusion of cranial sutures. Premature fusion also leads to reduced brain growth. Therefore, to reduce ICP and achieve a good clinical outcome by allowing normal brain growth, surgery is performed at an early age. Surgery for CS involves cranial vault expansion or remodeling.2
In this context, ICP monitoring is, therefore, likely to help in understanding the severity of CS and to evaluate the effectiveness of the surgical procedure.1 3 4 5 The measurement of ICP also helps guide the timing of surgery. ICP monitoring is useful in the management of children with CS to evaluate the need to normalize ICP and thereby reduce the risk of long-term sequelae of ICP elevation. Since the clinical features or radiological findings are not accurate determinants of increased ICP, monitoring using extradural, subdural, and parenchymal monitors would provide reliable information about ICP.1 Intraoperative ICP monitoring is helpful in guiding the correction of overall shape, cranial vault expansion, and height reduction for the correction of abnormal turret shape of the skull in the case of bicoronal synostosis.2 6
One of the problems of ICP monitoring in children with CS is the fact that there is no universally accepted scale of normal and abnormal ICP values in the pediatric age. Minns' scale for normal ICP values according to age cannot be adapted to children with CS.7 Most authors state that children with CS should be evaluated according to adult parameters (normal ICP range below 10 mm Hg, borderline 10–15 mm Hg, and abnormal above 15 mm Hg).8
In this study, we analyzed the utility of ICP monitoring intraoperatively for deciding height reduction and need for cerebrospinal fluid (CSF) diversion during cranial vault remodeling in children with multisutural CS. This study comprises of children with the involvement of two or more sutures. The ICP monitoring was not done for children with single suture involvement.
Methods
We conducted a retrospective observational study of children who underwent surgery for CS at our hospital. A waiver of consent was obtained from the Institute Ethics Committee. From the prospectively collected database of children with CS, the data of children who underwent ICP monitoring during surgery over the past 11 years (2009–2020) were retrieved. The following data were collected from the medical records of these patients: demographics including age, sex and weight, diagnosis (the type of CS), presence of any syndrome related to CS, presence of symptoms and signs of increased ICP, the surgical procedure performed, and findings on radiological imaging. The data of the following clinical features, which are indirect indicators of increased ICP, were collected: papilledema, vision impairment, proptosis, prominent veins over the scalp, and signs and symptoms of upper airway obstruction. The data of imaging findings included were sutures involved, presence of hydrocephalus, presence of Chiari-1 malformation, prominent emissary veins, venous sinus stenosis, and effacement of basal cisterns.
Anesthesia Protocol
All the children in this study were administered muscle relaxant and ventilated with volume control mode intraoperatively. The minute ventilation had been adjusted to maintain an end-tidal carbon dioxide (EtCO2) between 30 and 35mm Hg. Inhalational anesthestic, sevoflurane, was used uniformly for the maintenance of anesthesia. The depth of anesthesia was not monitored; however, the minimum alveolar concentration (MAC) was maintained between 0.8 and 1 MAC. This is also the usual anesthesia practice in our institute. Intraoperatively, all patients were positioned with head in neutral position. The systolic blood pressure target was 80 mm Hg in infants and more than 90 mm Hg in children over 1 year of age, and whenever a drop in blood pressure was observed, a fluid bolus or a bolus of 1.5 mg of mephentermine was administered.
Surgical Procedure for Bicoronal Synostosis
All children underwent surgery in the supine position (Fig. 1). The surgery was done as described by Persing et al.2 After anterior and posterior supraperiosteal scalp dissection extending from orbital rim to superior nuchal line, a ventricular catheter was inserted and opening ICP was recorded. Bilateral frontal craniotomy and bilateral orbitotomy were done. Bilateral parieto-occipital craniotomy was done leaving bone over the vertex of the skull and two lateral struts of bone extending from the vertex to the bilateral temporal bone on both sides. Fronto-orbital advancement was done. Multiple radial staves were made in the parieto-occipital skull flap, which was tied with dura over the occipital lobe. The parieto-occipital bone was not tied to the skull. This procedure increased the anteroposterior diameter of the skull. The bone struts extending from the vertex of the skull to the temporal bone were divided and reduced in height (approximately 1 cm) slowly under ICP monitoring. The ICP increased transiently during height reduction. The strut of the vertex bone was then fixed to the temporal bone. The skin was closed after inserting a subgaleal drain.
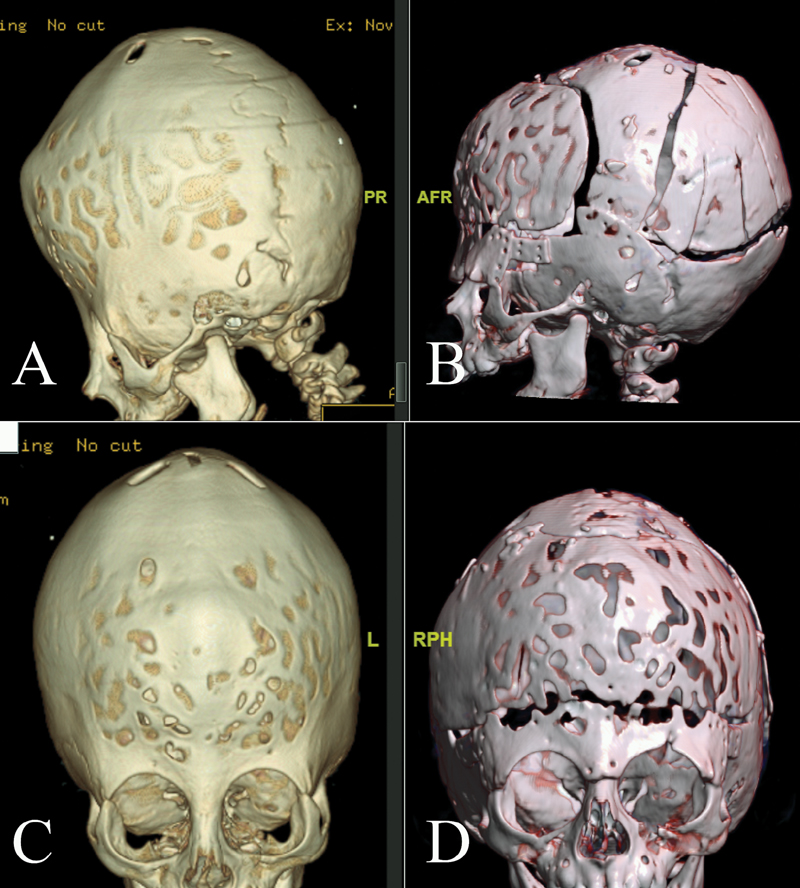
-
Fig. 1 Preoperative and postoperative CT scans of a case of bicoronal synostosis showing the bone cuts and height reduction. (A) and (C) preoperative lateral and anteroposterior view. (B) and (D) postoperative lateral and anteroposterior view.
Fig. 1 Preoperative and postoperative CT scans of a case of bicoronal synostosis showing the bone cuts and height reduction. (A) and (C) preoperative lateral and anteroposterior view. (B) and (D) postoperative lateral and anteroposterior view.
Surgical Procedure for Other CS
Children with normocephalic pancraniosynostoses who presented late with vision impairment underwent only ventriculoperitoneal (VP) shunt. Children with pancraniosynostoses who had proptosis underwent fronto-orbital advancement, cranial vault expansion, and remodeling. The cranial vault remodeling surgery was customized for other multisutural CS addressing the disfiguring deformity.
ICP Monitoring
After raising the bifrontal supraperisoteal scalp flap, a burr hole was made at right Kocher's point in the frontal skull (Fig. 2). Normal levels of PaCO2 and blood pressure for age was ensured before ventricular catheter insertion. External ventricular drainage (EVD) catheter was inserted and connected to the arterial pressure transducer, which was connected to a multiparameter monitor. Neuronavigation was used for children with very small ventricles. After obtaining the ICP waveform, the mean ICP was recorded. An abnormal value of ICP was defined as ICP >15 mm Hg.8 ICP monitoring was continued during the entire procedure. The ventricular catheter was removed at the time of closure. If the ICP remained elevated even at the time of closure, the EVD was retained in the postoperative period for a maximum of 5 days. Once ICP normalized, the EVD was removed. However, if ICP remained persistently elevated, then EVD was converted to a VP shunt.
-
At the time of insertion
-
After craniotomy
-
After height reduction
-
At closure
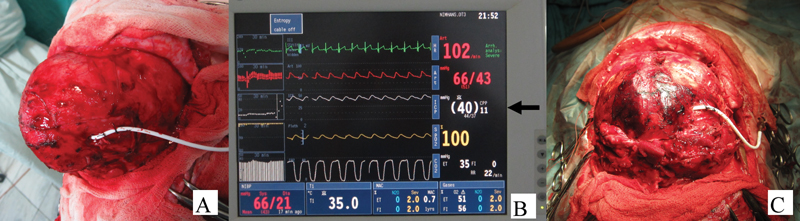
-
Fig. 2 Intraoperative pictures of a case of bicoronal craniosynostosis. (A) External ventricular catheter at Kocher's point. (B) Opening ICP, 40 mm Hg (arrow). (C) After craniotomy and height reduction before closure, ICP, 12 mm Hg (not shown).
Fig. 2 Intraoperative pictures of a case of bicoronal craniosynostosis. (A) External ventricular catheter at Kocher's point. (B) Opening ICP, 40 mm Hg (arrow). (C) After craniotomy and height reduction before closure, ICP, 12 mm Hg (not shown).
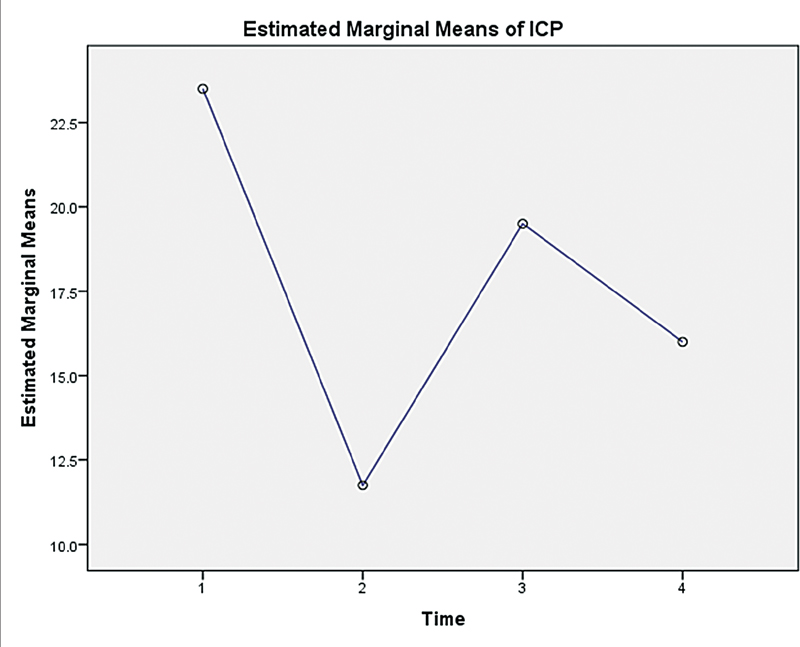
The ICP values were noted at the following periods (Fig. 3):
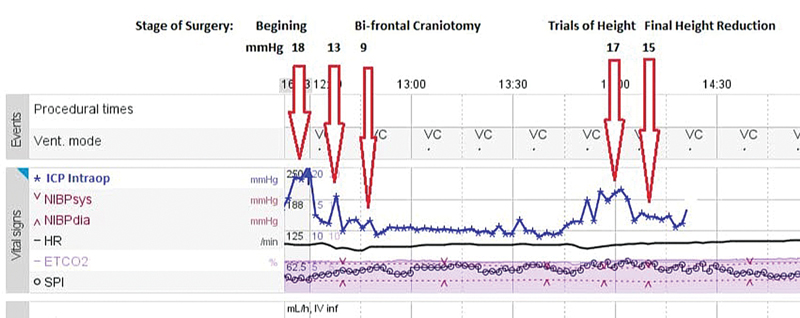
-
Fig. 3 An illustration of ICP trend during different steps of surgery.
Fig. 3 An illustration of ICP trend during different steps of surgery.
Statistical Analysis
Repeated measures analysis of variance (RM ANOVA) was performed to test the difference in ICP of subjects across the four-time points. Initially, the data were checked for the appropriateness of the test by evaluating the required assumptions. The first three assumptions were fulfilled as the outcome variable, i.e., ICP was continuous with more than two matched measurement time points and with no significant outliers in each time point. The assumption of normality was evaluated using Kolmogorov–Smirnova and Shapiro–Wilk tests. The assumption of sphericity, which says that the variances of the differences between all combinations of related groups must be equal, was checked using Mauchly's test. The Huynh–Feldt test was used to evaluate the difference in ICP at various time points. Although the data followed all the assumptions required for performing RM ANOVA, it was a matter of concern that the overall number was small; hence, a non-parametric analog of RM ANOVA viz., Friedman test, was performed to demonstrate a significant difference in ICP across the time points.
Results
A total of 28 (19 boys) children with the involvement of two or more sutures underwent ICP monitoring during surgery. The age of these children ranged from 5 months to 9 years, 14 (50%) were infants aged less than 1 year. The commonest pattern of suture involvement was bicoronal seen in 16 (57.1%) children followed by pancraniosynostoses in eight (28.6%) cases. Fourteen (50%) children had syndromic CS. Fifteen (53.6%) children had subnormal development. On evaluation with a computed tomography scan of the head, the copper beaten appearance was seen in 18 (64.3%) cases. The magnetic resonance imaging revealed Chiari type-1 malformation in 14 (50%) cases (Table 1).
|
Age in months, median (range) |
12.5 (5–108) |
|---|---|
|
Boys: Girls |
19:9 |
|
Sutures involved • Bicoronal • Unicoronal and metopic • Bilateral lambdoid and sagittal • Pancraniosynostosis |
16 (57.1%) 1 (3.6%) 3 (10.7%) 8 (28.6%) |
|
Syndromes • Apert • Crouzon • Saethre–Chotzen • None |
5 (17.9%) 8 (28.6%) 1 (3.6%) 16 (57.1%) |
|
Clinical features • Papilloedema • Vision impairment • Proptosis • Prominent veins on scalp • Upper airway obstruction • Subnormal development • Social quotient (mean +/− SD) |
8 (28.6%) 6 (21.4%) 21 (75%) 3 (10.7%) 7 (25%) 15 (53.6%) 86.9 (+/− 23.9) |
|
Imaging findings • Copper beaten appearance on CT scan • Large emissary veins • Stenosis of sigmoid sinus • Chiari 1 malformation |
18 (64.3%) 4 (14.3%) 4 (14.3%) 14 (50%) |
|
Surgical procedure • Cranial vault remodeling • VP shunt • VP shunt with cranial vault remodeling |
22 (78.6%) 4 (14.3%) 2 (7.1%) |
Abbreviations: CT, computerized tomography; SD, standard deviation; VP, ventriculoperitoneal.
Cranial vault remodeling was done for 22 (78.6%) cases. Two (7.1%) children also underwent VP shunt following cranial vault remodeling for persistently elevated ICP. Four (14.1%) children underwent only VP shunt for normocephalic pancraniosynostoses. The children with pancraniosynostoses presented late and had a largely normal shaped head; hence, only VP shunt was done.
Intracranial Pressure
The mean opening ICP was 23 mm Hg, ranging from 7 to 40 mm Hg. Eighteen (64.3%) children had high (>15 mm Hg) opening pressure. The mean opening ICP in children who underwent only VP shunt was 24.2 mm Hg, ranging from 19 to 29 mm Hg. The mean opening ICP in children who underwent cranial vault remodeling was 22.7 mm Hg, ranging from 7 to 40 mm Hg. The ICP dropped to 10.9 mm Hg, ranging from 3 to 21 after craniotomy. The ICP increased transiently to 19.5 mm Hg, ranging from 15 to 25 after height reduction to correct turret deformity. The ICP was 16.2 mm Hg, ranging from 9 to 21 mm Hg at the time of closure (Table 2, Fig. 4). The result of the Friedman test showed a significant difference in ICP across time points (χ2 = 8.189, p-value = 0.042) (Fig. 5).
|
Initial ICP |
22.7 (9.4), [7–40] |
|
ICP after craniotomy |
10.9 (5.8), [3–21] |
|
ICP after height reduction |
19.5 (4.1), [15–25] |
|
ICP at closure |
16.2 (3.4), [9–21] |
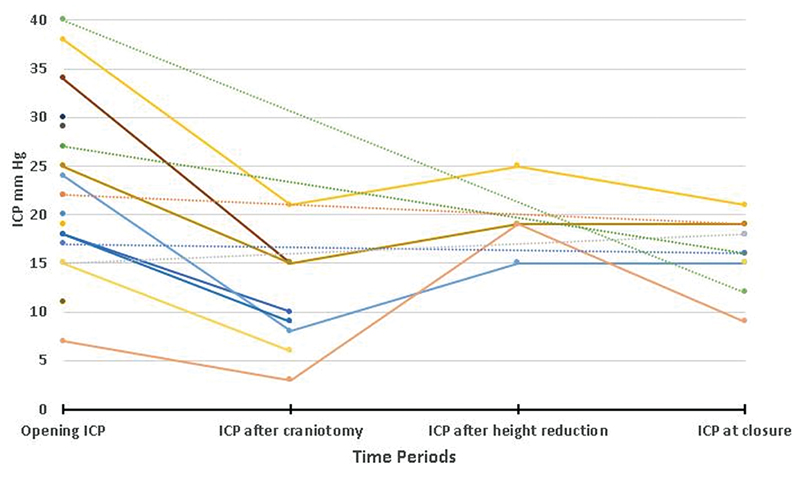
-
Fig. 4 Graph showing trend in ICP at different periods after surgery for individual cases.
Fig. 4 Graph showing trend in ICP at different periods after surgery for individual cases.
-
Fig. 5 Estimated marginal mean plots for the cases with ICP values at following time periods: (1) At the time of insertion, (2) After craniotomy, (3) After height reduction, and (4) At closure.
Fig. 5 Estimated marginal mean plots for the cases with ICP values at following time periods: (1) At the time of insertion, (2) After craniotomy, (3) After height reduction, and (4) At closure.
In two children, the ICP remained persistently elevated to more than 20 mm Hg after height reduction; hence, the stitches of vertex bone strut and temporal bone were opened after which ICP normalized. In four children, the ICP was marginally high, i.e., 15 to 20 mm Hg. One child underwent a VP shunt in the same sitting as there was significant ventriculomegaly, and the EVD was retained during the postoperative period for ICP monitoring in three children. The ICP normalized in two out of three children in the postoperative period. One child who had persistently elevated ICP in the postoperative period underwent VP shunt. None of the children had any complications related to EVD.
Discussion
Elevation of the ICP has been reported in more than half of the children with multisutural CS. The prevalence of increased ICP in multisutural craniosynostosis is 58 to 67% and around 31% for bicoronal synostosis.6 Intracranial hypertension in children with CS is often associated with a slowly progressive clinical course. The majority of children have no warning signs or symptoms and may present at an advanced stage of the disease.8 Half of the children in our series were older than 1 year of age. It is due to the referral pattern. In our country, the awareness about craniosynostosis is still low. Unless the head is significantly deformed or the child has additional neurological symptoms, most parents do not seek neurosurgical consultation. In our series, a few children had normocephalic pancraniosynostosis and sought consultation for symptoms of increased ICP like vision impairment at an advanced age. Surgery was offered to all the parents at the time of presentation in outpatient department. However, due to variable age at presentation, the age of these children ranged from 5 months to 9 years and only 14 (50%) children were aged less than 1 year at the time of surgery. The correspondence between ICP values and clinical findings like papilledema, vision impairment, intellectual delay, etc., or radiological findings like ventriculomegaly, intracranial volume, basal cisterns, copper beaten appearance, etc., related to ICP abnormalities is not reliable.9 10 11 12 13 The ICP monitoring was done in all children with syndromic CS as well as in cases with multisuture involvement in our series. The ICP monitoring of children with CS may help in deciding the indication and timing of surgery. However, the lack of cooperation of younger children may influence the reliability of the ICP recordings.8 Yokote et al suggested considering only monitoring of the ICP in the perioperative period.3 In our institute, we have been monitoring the ICP during surgery for multisutural CS. The main purpose of ICP monitoring during surgery was to document the presence of intracranial hypertension, change in ICP after surgery, and guide the type of surgical procedure based on ICP recordings during surgery. Although there is no universally accepted threshold of ICP values for defining intracranial hypertension in children with CS, a cutoff of 15 mm Hg has been suggested.8 14 15 16 In our cohort, we found that more than half of the children with multisutural CS had elevated ICP. The proportion of children with increased ICP was higher in our series and also the proportion of syndromic cases was higher in our series. Increasing age and multisutural involvement are associated with higher incidence of increased ICP.17 As syndromic cases have multisutural involvement, we had a higher proportion of syndromic cases in our series. The ICP monitoring was found useful in our cases of bicoronal synostoses at the time of reducing the height of the vertex to correct the turret deformity. It has been observed that the reshaping of the cranial vault and height reduction may lead to a temporary elevation in ICP but within a short period, the removal of confining bone reduces the ICP overall.2 In our cohort, we found that the height reduction could not be achieved as desirable because of persistently elevated ICP in two children. In four children, the ICP did not normalize after cranial vault remodeling and height reduction, but it was still lower than pre-craniotomy level; hence, an EVD was left behind, and ICP monitoring was continued in the postoperative period. We have not done rigid bone fixation using metallic screws and plates in any case. This gave the scope of further cranial vault remodeling and dural expansion in the postoperative period leading to reduction of ICP. This was observed in two cases, and EVD could be removed. However, two children had persistently elevated ICP in the postoperative period. These children underwent VP shunt. One child had hydrocephalus, and ICP did not come down despite cranial vault remodeling; hence, a VP shunt was done. Another child had persistent increased ICP, which did not come down even after EVD in the postoperative period; hence, VP shunt was done. The EVD or a VP shunt may prevent brain expansion, but in children with intracranial hypertension who do not respond to cranial vault remodeling, it may not be possible to wait for a prolonged period for ICP reduction. In the absence of ICP monitoring and VP shunt in the above cases, the children would have suffered from intracranial hypertension for an indefinite period after surgery. These children might have developed clinical signs and symptoms of intracranial hypertension like papilloedema, vision impairment, and developmental delay. It is difficult to say whether the decision to do shunt was premature as we do not have enough data from our study and from the literature on the timing of normalization of ICP after surgery. We observed that in two children though the ICP was high in the immediate postoperative period it normalized within 5 days, whereas in the other two children it did not. Keeping an EVD for more than 5 days incurs a significant risk of infection. In that case scenario, we felt it was safer to convert to a VP shunt. We have not found any complications related to VP shunt like subdural hematoma/hygroma in our cohort. Telemetric ICP monitoring might have been useful for monitoring these children.18 Currently, we do not have the facility of telemetric ICP monitoring in our country. The ICP normalized after surgery in the rest of the children, and they did not require further surgical procedures or modifications of procedure.
We also did VP shunt to reduce the ICP of children with pancraniosynostosis. Children with pansutural involvement have two types of presentation. One group presents with normal shaped head (normocephalic craniosynostosis) and some amount of ventriculomegaly. These children present late, after the growth of the brain is complete. These children present with symptoms of increased ICP like vision impairment due to papilloedema and secondary optic atrophy. Patients with normocephalic pancraniosynostosis have an insidious clinical course. Because of their indistinct cranial morphology, they present late with significant symptoms of elevated ICP requiring surgery to reduce ICP.19 Only VP shunt was done in such cases. We have found stabilization of vision or improvement after VP shunt. The second group with pancraniosynostosis present with abnormally shaped head, particularly syndromic cases. These children present with proptosis and other features of syndrome. Orbital advancement and cranial vault remodeling were done for such cases.
A limited number of studies have reported preoperative and postoperative ICP monitoring in children with CS. ICP monitoring may play an important role in the postoperative evaluation of children with CS who have undergone surgery.8 Siddiqi et al reported episodes of increased ICP in 6% of 107 children who underwent surgery for CS. All children had an identifiable syndrome. The clinical evidence of increased ICP was found 2 to 5 years after surgery.20 Thompson et al monitored ICP in 22 children with syndromic CS 1 to 3 months after surgery and found increased ICP in 5% of children.21 These studies suggest that children with complex CS should undergo regular follow-up for causative factors of intracranial hypertension hydrocephalus, impairment of venous return, and upper airway obstruction.
A significant reduction in mean ICP or the number of ICP elevations immediately after surgery has been reported.3 16 In a study by Tamburrini et al 11 out of the 12 children with complex CS had abnormal ICP values in the preoperative period. Six patients who underwent immediate postoperative monitoring showed a significant reduction in ICP. The mean postoperative length of ICP monitoring was 42.1 hour.22 Tamburrini et al recommended that evaluation of ICP recordings should be prolonged for at least 24 hours after surgery. The ICP monitoring was done for 5 days for children who did not have a reduction of ICP after cranial vault remodeling in our series. Eide et al tested the number of episodes of ICP more than 20 mm Hg before and after surgery in 18 children. Eleven children showed pathological findings. Postoperatively, the number of ICP elevations significantly reduced in all patients (p < 0.005), though mean ICP did not change significantly.23 Yokote et al monitored ICP intraoperatively in six children. They recorded ICP values before and after craniotomy. The mean pre-and postoperative ICPs were 14.7 and 4.2 mm Hg, respectively.3
The updated Dutch guideline on the treatment and management of craniosynostosis recommends to treat increased ICP based on patient-specific causal factors, i.e., a too-small intracranial volume, moderate-to-severe obstructive sleep apnea, hydrocephalus, and venous intracranial hypertension. The guidelines have also recommended several options for treatment, for example, cranial expansion surgery, a VP shunt, or an endoscopic third ventriculostomy. We did cranial vault remodeling in 78.6%, only VP shunt in 14.3%, and VP shunt following cranial vault remodeling in 7.1% cases.6
Conclusion
We demonstrated the utility of ICP monitoring during surgery for guiding the extent of surgery, type of surgery, and need for further surgical procedures. The ICP monitoring led to the modification of surgical procedures in one-eighth of our patients. The perioperative ICP monitoring also helped to define the indication of VP shunt in one-eighth of our patients after cranial vault remodeling. Based on our findings, we suggest conducting further studies on monitoring of ICP in the perioperative period for children with multisutural CS. There is a need for defining clear guidelines on the protocol of ICP measurement in children with CS.
Conflict of Interest
None declared.
Funding None.
References
- Intraoperative intracranial pressure monitoring in the pediatric craniosynostosis population. J Neurosurg Pediatr. 2018;22(5):475-480.
- [Google Scholar]
- Craniosynostosis.Youmans Neurological Surgery. Philadelphia: Elsevier; 2011. p. :1940-1954. In: ed.
- [Google Scholar]
- Intraoperative pre- and post-craniofacial reconstruction intracranial pressure (ICP) monitoring in children with craniosynostosis. Childs Nerv Syst. 2013;29(8):1363-1367.
- [Google Scholar]
- Use of neuroimaging measurements of optic nerve sheath diameter to assess intracranial pressure in craniosynostosis. Childs Nerv Syst. 2018;34(5):939-946.
- [Google Scholar]
- Intracranial volume versus static and pulsatile intracranial pressure values in children with craniosynostosis. J Neurosurg Pediatr. 2019;24(1):66-74.
- [Google Scholar]
- Updated guideline on treatment and management of craniosynostosis. J Craniofac Surg. 2021;32(1):371-450.
- [Google Scholar]
- ICP in craniosynostosis.Textbook of Pediatric Neurosurgery. Cham: Springer International Publishing; 2018. p. :1-8. In:
- [Google Scholar]
- The preoperative incidence of raised intracranial pressure in nonsyndromic sagittal craniosynostosis is underestimated in the literature. J Neurosurg Pediatr. 2014;14(6):674-681.
- [Google Scholar]
- Nocturnal ultrasound measurements of optic nerve sheath diameter correlate with intracranial pressure in children with craniosynostosis. Plast Reconstr Surg. 2012;130(3):448e-451e.
- [Google Scholar]
- The relationship between intracranial pressure and size of cerebral ventricles assessed by computed tomography. Acta Neurochir (Wien). 2003;145(3):171-179. , discussion 179
- [Google Scholar]
- The effectiveness of papilledema as an indicator of raised intracranial pressure in children with craniosynostosis. Neurosurgery. 1996;38(2):272-278.
- [Google Scholar]
- Enigma of raised intracranial pressure in patients with complex craniosynostosis: the role of abnormal intracranial venous drainage. J Neurosurg. 2001;94(3):377-385.
- [Google Scholar]
- Intracranial pressure monitoring in children: comparison of external ventricular device with the fiberoptic system. Childs Nerv Syst. 1993;9(8):470-473.
- [Google Scholar]
- Results and complications of intracranial pressure monitoring in 303 children. Pediatr Neurosurg. 1995;23(2):64-67.
- [Google Scholar]
- Assessment of continuous intracranial pressure recordings in childhood craniosynostosis. Pediatr Neurosurg. 2002;37(6):310-320.
- [Google Scholar]
- Elevated intracranial pressure with craniosynostosis: a multivariate model of age, syndromic status, and number of involved cranial sutures. J Neurosurg Pediatr 2021 (e-Pub ahead of print).
- [CrossRef] [Google Scholar]
- Telemetric intracranial pressure monitoring in children. Childs Nerv Syst. 2020;36(1):49-58.
- [Google Scholar]
- Normocephalic pancraniosynostosis resulting in late presentation of elevated intracranial pressures. Plast Reconstr Surg. 2010;125(5):1493-1502.
- [Google Scholar]
- The detection and management of intracranial hypertension after initial suture release and decompression for craniofacial dysostosis syndromes. Neurosurgery. 1995;36(4):703-708. , discussion 708–709
- [Google Scholar]
- Intracranial pressure in single-suture craniosynostosis. Pediatr Neurosurg. 1995;22(5):235-240.
- [Google Scholar]
- Intracranial pressure monitoring in children with single suture and complex craniosynostosis: a review. Childs Nerv Syst. 2005;21(10):913-921.
- [Google Scholar]
- A computer-based method for comparisons of continuous intracranial pressure recordings within individual cases. Acta Neurochir (Wien). 2003;145(5):351-357. , discussion 357–358
- [Google Scholar]







