Translate this page into:
At the Eye of the Hurricane! Perioperative Management of an Unoptimized Metastatic Pheochromocytoma Presenting for Emergency Neurosurgery
Ajay Prasad Hrishi, MD, DM, EDAIC Department of Neuroanesthesia, Sree Chitra Tirunal Institute for Medical Sciences and Technology Thiruvananthapuram, Kerala 695011 India drajay@sctimst.ac.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Metastatic pheochromocytoma (PCC) is a rare entity arising from extra-adrenal tissue. We report the perioperative management of a young woman presenting with metastatic PCC to the vertebral body resulting in vertebral collapse and spinal cord compression necessitating emergency surgery. There are no reports of anesthetic management of a patient with unoptimized metastatic PCC presenting for emergency neurosurgery under general anesthesia. Our anesthetic goals were to maintain a deep anesthetic plane with stable hemodynamics, facilitate intraoperative neuromonitoring, manage catecholamine surges during anesthetic induction, tumor resection, and manage perioperative massive blood loss. The successful perioperative management of metastatic PCC has become possible with the vast armamentarium of anesthetic drugs and intraoperative advanced monitoring techniques. In addition, our role in understanding the pathophysiology and course of the disease is essential to ensure low morbidity and mortality of such cases in their most vulnerable perioperative period.
Keywords
metastatic pheochromocytoma
neurosurgery
intraoperative neuromonitoring
Introduction
Pheochromocytoma (PCC) is a rare neuroectodermal tumor arising from chromaffin cells in the adrenal medulla with an incidence of 2 to 8 per million.1 Extra-adrenal PCC or paragangliomas (incidence of 1–2 per million) is a rarer entity that arises from extra-adrenal tissue, of which 40 to 50% are metastatic.2 We report the perioperative management of a young female presenting with metastatic PCC to the vertebral body resulting in vertebral collapse and spinal cord compression necessitating emergency surgery. There are no reports of anesthetic management of an unoptimized metastatic PCC presenting for emergency neurosurgery under general anesthesia.
Case History
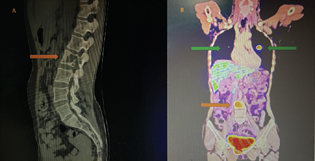
A 40-year-old, malnourished woman with a body mass index of 14.5 kg/m2 presented to the emergency department with features suggestive of impending cauda equina. Imaging with computed tomography scan of the spine which revealed an expansile lytic lesion in the lumbar L2 vertebral body extending into the spinal canal, causing spinal cord compression with similar lytic lesions was noted in the inferior pubic ramus (Fig. 1A). A whole-body positron emission tomography scan revealed hypermetabolic lesions in the vertebral body, bilateral lungs, and increased fluorodeoxyglucose uptake in the ileocolic nodes, right iliac fossa, suggestive of metastatic PCC (Fig. 1B). She gave a history of surgical excision of adrenal PCC 13 years ago and was on Tab. metoprolol 25 mg twice daily for the past 12 years. However, she had poor compliance with the medication and follow-up medical evaluation. On examination, her heart rate (HR) was 120 bpm, and her blood pressure (BP) was 170/80. Laboratory investigations revealed hypokalemia with a serum potassium of 1.9 mEq/L, hyperglycemia, and increased urinary normetanephrines and urinary vanillylmandelic acid levels. The rest of the laboratory parameters were within normal limits. Preoperative electrocardiogram (ECG) showed high-voltage complexes and signs of left ventricular hypertrophy (LVH). Echocardiography revealed hyperdynamic circulation with concentric LVH and an ejection fraction of 75%.
-
Fig. 1 (A) Figure showing computed tomography of the spine showing lytic lesion in the lumbar L2 vertebral body extending into the spinal canal causing spinal cord compression. (B) Positron emission tomography scan showing hypermetabolic lesions in the vertebral body (orange arrow), bilateral lungs (green arrow), and increased fluorodeoxyglucose uptake in the ileocolic nodes, right iliac fossa.
Fig. 1 (A) Figure showing computed tomography of the spine showing lytic lesion in the lumbar L2 vertebral body extending into the spinal canal causing spinal cord compression. (B) Positron emission tomography scan showing hypermetabolic lesions in the vertebral body (orange arrow), bilateral lungs (green arrow), and increased fluorodeoxyglucose uptake in the ileocolic nodes, right iliac fossa.
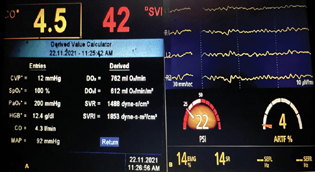
The patient was posted for emergency spinal cord decompression under general anesthesia in view of impending cauda equina. Emergency endocrine consultation was sought, and an antihypertensive regimen of Tab. prazosin 5 mg twice daily along with Tab. amlodipine 5 mg twice daily was advised. However, the antihypertensive therapy could not be optimized due to the emergent nature of the surgery. Preoperatively, the patient was transferred to a high-dependency unit and given 60 mmol of 10% potassium chloride at a rate of 10 mmol/h with ECG monitoring to correct the hypokalemia. The patient was premedicated with Tab. diazepam 5 mg before surgery, and a eutectic mixture of local anesthesia (EMLA) was applied at the site of intended peripheral venous and radial arterial cannulation. Preinduction monitors included ECG, pulse oximetry (SPO2), invasive BP, and patient state index (PSI) with Sedline (Root O3, Masimo Corporation; Irvine, California, United States) for depth of anesthesia. FloTrac (version 3.06, Edwards Life Science, Irvine, California, United States) was transduced with the arterial line to monitor beat-to-beat cardiac output (CO), stroke volume variation (SVV), and systemic vascular resistance (SVR) (Fig. 2A).
-
Fig. 2 (A) FloTrac monitor showing the beat-to-beat cardiac output (CO), stroke volume (SV), stroke volume index (SV), stroke volume index (SVI), cardiac index (CI), systemic vascular resistance (SVR), and the derived values of global oxygen delivery (DO2), global oxygen delivery index (DO2I), and systemic vascular resistance index (SVRI). (B) Figure showing the Sedline (Root O3, Masimo Corporation; Irvine, California, United States) depth of anesthesia monitor showing frontal electroencephalographic and patient state index values.
Fig. 2 (A) FloTrac monitor showing the beat-to-beat cardiac output (CO), stroke volume (SV), stroke volume index (SV), stroke volume index (SVI), cardiac index (CI), systemic vascular resistance (SVR), and the derived values of global oxygen delivery (DO2), global oxygen delivery index (DO2I), and systemic vascular resistance index (SVRI). (B) Figure showing the Sedline (Root O3, Masimo Corporation; Irvine, California, United States) depth of anesthesia monitor showing frontal electroencephalographic and patient state index values.
Inj. magnesium 1.5 g was given intravenously over 5 minutes. After preoxygenation with 100% fraction of inspired oxygen for 3 minutes, anesthesia was induced with fentanyl 2 µg/kg and etomidate 0.5 mg/kg titrated to PSI of 25 to 30. Tracheal intubation was facilitated with vecuronium 0.1 mg/kg and Inj. labetalol 5 mg was administered 3 minutes before intubation. Hemodynamics remained stable throughout the anesthetic induction. Anesthesia was maintained on total intravenous anesthesia (TIVA) with propofol 150 µg/kg/min, fentanyl 2 µg/kg/h, and dexmedetomidine 0.5 µg/kg/h titrated to a PSI of 20 to 25 (Fig. 2B). TIVA was preferred to facilitate intraoperative neuromonitoring with triggered electromyography (EMG) monitoring of sphincter and lower limbs. Postinduction, central venous access was obtained in the right internal jugular vein under ultrasound guidance. It was primed with noradrenaline and sodium nitroprusside (SNP) to manage the hemodynamic fluctuations throughout the surgery. Before prone positioning, intravascular volume status was optimized with 4 mL/kg isotonic crystalloid titrated to SVV <10%.
A surgical incision was made from the D12–L5 spinal levels. Intraoperatively, during surgical excision of the tumor, the BP increased to > 20% of the baseline but was effectively managed by raising the dexmedetomidine to 0.7 µg/kg/h. However, postresection of the tumor patient had a hypotensive episode which was refractory to noradrenaline at 2 µg/kg/min. At this juncture, the HR was 70 bpm, BP was 80/50 mm Hg, CO was 3 L/min, SVV was 12%, and SVR was 600 dynes/s/cm5. This prompted us to initiate vasopressin infusion, and normotension was achieved at a dose of 0.02 U/min. Following resection, an epidural catheter was placed by surgeons at the L1 level, and epidural infusion of levobupivacaine 0.125% + fentanyl 4 µg/mL was started at 10 mL/h. The surgery lasted for 5 hours with an approximate blood loss of 1,200 mL, which was replaced. Postoperatively, the patient was extubated uneventfully and shifted to a high-dependency unit for postoperative care. Postoperative analgesia was successfully managed with a multimodal approach consisting of epidural analgesia and Inj. paracetamol 20 mg/kg 8 hourly. The postoperative course was uneventful, and the patient was discharged from the hospital on the fifth postoperative day.
Discussion
Anesthetic management of metastatic PCC is a challenging scenario. The perioperative management of our case was challenging as the patient was unoptimized, malnourished, and presented for emergency neurosurgery in the prone position. Unoptimized PCC presenting for surgery is known to have high perioperative mortality of up to 45%. The reasons are severe intraoperative hypertension, myocardial ischemia, left ventricular failure, arrhythmias, strokes, and refractory hypotension after tumor resection.3
Our anesthetic goals were to maintain a deep anesthetic plane with stable hemodynamics, facilitate intraoperative neuromonitoring, manage catecholamine surges during anesthetic induction, intubation, tumor resection, and manage perioperative massive blood loss. Conventionally, in patients presenting with PCC, the BP is optimized before surgery. The alpha blockade commenced at least 1 to 2 weeks before surgery with a more prolonged course in patients with refractory hypertension.3 However, in our case, as an emergency surgery was warranted, we took the patient after administering a single dose of prazosin and amlodipine. Patients with PCC are prone to resistant hypokalemia due to high circulating epinephrine levels.3 Moreover, in such patients, there is concomitant hyperaldosteronism leading to contraction alkalosis, increased potassium excretion in urine, and excessive β2 stimulation resulting in the internalization of potassium.4
Preoperatively, to reduce the catecholamine surge due to anxiety and pain, the patient was administered anxiolytic along with preparation of intended invasive venous and arterial puncture sites with EMLA cream. Hyperglycemia was managed perioperatively with variable rate regular insulin infusion to target 140 to 180 mg/dL blood glucose. Hyperglycemia results from increased, impaired insulin release and increased glucagon release in conjugation with peripheral insulin resistance.3
In view of hyperdynamic circulation and concentric LVH to ensure hemodynamic stability during anesthetic induction, a titrated induction with etomidate, high-dose fentanyl, and vecuronium was preferred. Succinylcholine is better avoided as it is known to cause sympathetic activation resulting in a catecholamine surge in patients with PCC.3 Though atracurium is the preferred muscle relaxant in the setting of intraoperative EMG monitoring, we avoided it as it can cause a catecholamine surge secondary to histamine release.3 We preferred TIVA over inhalational because of intraoperative neuromonitoring and agents such as desflurane causes sympathetic stimulation, whereas halothane and sevoflurane sensitize the myocardium to the effects of catecholamines. In addition, laryngoscopy and endotracheal intubation cause a hypertensive crisis which is exaggerated in patients with extra-adrenal PCC.5 In our case, this response was attenuated by maintaining a deep plane of anesthesia, labetalol before laryngoscopy, and infusion of magnesium sulphate 1.5 g. As the alpha blockade was not optimized in our patient, we used labetalol instead of esmolol to avoid unopposed alpha action caused by esmolol. However, it should be borne in mind that labetalol may cause paradoxical episodes of hypertension as it has a fixed ratio of α- to β-antagonistic activity (1:7), and the α- to β-antagonistic activity should be at least 4:1 to achieve adequate antihypertensive effect. There has been robust evidence of the use of magnesium sulphate in PCC to maintain hemodynamics.3 It inhibits the release of catecholamines and reduces α-adrenergic receptor sensitivity to catecholamines. In addition, it has vasodilatory properties along with antiarrhythmic and membrane stabilizing properties making it an ideal adjuvant in this scenario.3
Patients with PCC are volume depleted due to uncontrolled hypertension, high catecholamines, and hyperaldosteronism. Moreover, our patient presented unoptimized and had to be positioned prone. Hence, we ensured adequate volume resuscitation with crystalloids before positioning guided by CO monitor (arterial pressure-based cardiac output [APCO]; FloTrac). Comparison of pulmonary artery-based CO (PACO) monitor versus arterial pulse-based CO monitor found the latter to be more effective. Furthermore, it was elucidated that PACO requires 6 minutes for averaging and displaying the CO, whereas APCO requires only 40 seconds.6 However, clinicians should exercise caution while interpreting the values from APCO as its specificity reduces in scenarios where there is high concentration of circulating catecholamines such as PCC.6 Moreover, it is challenging to predict volume status in such patients, especially in the prone position. SVV measured by FloTrac was found to more accurately predict volume status as it relied on arterial pressure fluctuations with respiration.6 An SVV of 14% is a reliable indicator of fluid responsiveness in the prone position.7
In our case, we effectively managed the hypertensive episodes with a combination of magnesium and dexmedetomidine. Dexmedetomidine is an ideal anesthetic adjuvant in the setting of intraoperative neuromonitoring owing to its sedative and analgesic properties. Additionally, dexmedetomidine substantially reduces the plasma norepinephrine levels via the central sympatholytic effects.8 This property made it an attractive agent in our scenario. Our patient did not develop hypertensive crisis during the tumor handling by the surgeon negating the use of SNP. However, the patient developed an episode of refractory hypotension postresection of the tumor and was unresponsive to norepinephrine. Hypotension is anticipated in the postresection phase of phaeochromocytoma due to the abrupt drop in the circulating catecholamines.3 9 Hypotension refractory to noradrenaline and phenylephrine has been reported in patients with adrenal and extra-adrenal PCCs.3 9 It is probably due to the downregulation of alpha and beta receptors due to long-term catecholamine receptor downregulation caused by chronic elevation of catecholamine levels.3 9 In contrast, vasopressin induces systemic vasoconstriction and pulmonary vasodilatation via the V1 receptor, in addition to increasing circulatory volume by increasing water reabsorption via V2 receptors in the kidney.9 However, even in this scenario of refractory hypotension, norepinephrine or epinephrine should be continued to ensure sustained chronotropy and inotropy when vasoconstriction is induced by vasopressin.
Postoperatively managing hemodynamic perturbation, pain, and glycemic control needs attention in this subset of patients.3 It is prudent that these patients receive high-dependency care with invasive arterial monitoring for at least 24 hours after the procedure.3 Hypertension is most commonly the result of pain, coexisting essential hypertension, urinary retention, or fluid overload. Moreover, rebound hyperinsulinism is known to occur in the immediate postoperative period due to the loss of inhibitory effect of catecholamines on insulin-producing cells.3 This can result in life-threatening hypoglycemia, thus warranting regular blood glucose monitoring.3 Effective pain management with adjuvant regional anesthesia offers hemodynamic stability and is essential.
Conclusion
The successful perioperative management of metastatic PCC has become possible with the vast armamentarium of anesthetic drugs and intraoperative advanced monitoring techniques. In addition, our role in understanding the pathophysiology and course of the disease is essential to ensure low morbidity and mortality of such cases in their most vulnerable perioperative period.
Conflict of Interest
None declared.
Funding None.
References
- Pheochromocytoma and paraganglioma: from epidemiology to clinical findings. Sisli Etfal Hastan Tip Bul. 2020;54(2):159-168.
- [Google Scholar]
- Perioperative management of metastatic paraganglioma-pheochromocytoma of the humerus with the aid of regional anesthesia. Case Rep Anesthesiol. 2020;2020:2482793.
- [Google Scholar]
- Pheochromocytoma secreting large quantities of both epinephrine and norepinephrine presenting with episodes of hypotension and severe electrolyte imbalance. Cureus. 2018;10(7):e3050.
- [Google Scholar]
- Risk factors for hemodynamic instability during surgery for pheochromocytoma. J Clin Endocrinol Metab. 2010;95(2):678-685.
- [Google Scholar]
- Intraoperative hemodynamics monitoring in a patient with pheochromocytoma multisystem crisis: a case report. JA Clin Rep. 2018;4(1):35.
- [Google Scholar]
- Accuracy of stroke volume variation and pulse pressure variation to predict fluid responsiveness in patients with thoracic kyphosis. Ann Palliat Med. 2021;10(7):7571-7578.
- [Google Scholar]
- Dexmedetomidine induced catecholamine suppression in pheochromocytoma. J Nat Sci Biol Med. 2014;5(1):182-183.
- [Google Scholar]
- Use of vasopressin bolus and infusion to treat catecholamine-resistant hypotension during pheochromocytoma resection. Anesthesiology. 2007;106(4):883-884.
- [Google Scholar]






