Translate this page into:
Hirayama disease
Address for correspondence: Dr. Atul T. Tayade, Department of Radiodiagnosis, MGIMS, Sewagram, Wardha, Maharashtra-442102, India. E-mail: tayadeatul@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 17-year-old male, who gave up his favorite sport cricket and started playing football, presented with one-year history of slowly progressive atrophic weakness of forearms and hands. Neurological examination showed weak and wasted arms, forearms and hand but no evidence of pyramidal tract, spinothalmic tract and posterior column lesions. Plain cervical spine radiographs showed no abnormal findings. Cervical magnetic resonance imaging (MRI) showed asymmetric cord atrophy; images obtained with neck flexed showed the anterior shifting of the posterior wall of the lower cervical dural sac resulting in cord compression. These findings suggest Hirayama disease, a kind of cervical myelopathy related to the flexion movements of the neck.
Keywords
Amyotrophy
atopy
hirayama disease
Introduction
Hirayama disease, a rare neurological disease, is characterized by insidious unilateral or bilateral muscular atrophy and weakness of the forearms and hands, without sensory or pyramidal signs.[1] The disease primarily affects men in the second to third decades. The disease progresses initially, but spontaneous arrest is known to follow several years after the onset, unlike motor neuron disease with which it is commonly confused.[2] Hirayama disease is characterized by focal ischemic changes in the anterior horn cells of the lower cervical cord that result in amyotrophy, which is usually unilateral but may also be bilateral. We describe the characteristic findings of flexion magnetic resonance imaging (MRI) suggestive of Hirayama disease.
Case Report
A 17-year-old male reported in the medicine out patient clinic with a one year history of slowly progressive weakness and atrophy that started in the left hand and the forearm and gradually involved the right hand. The hand weakness limited several activities of his daily living and he could no longer play cricket because of the same. He developed clawing of both hands, but had normal sensations. His past medical history was noncontributory; he denied any allergic disease and none of his family members had similar symptoms. Neurological examination revealed severely wasted and weak forearms and hands, tremors but no evidence of pyramidal, spinothalmic, posterior column lesions, polyminimyoclonus or autonomic disturbances.
Plain cervical spine radiographs showed no abnormalities, or misalignment of the vertebral bodies [Figure 1].
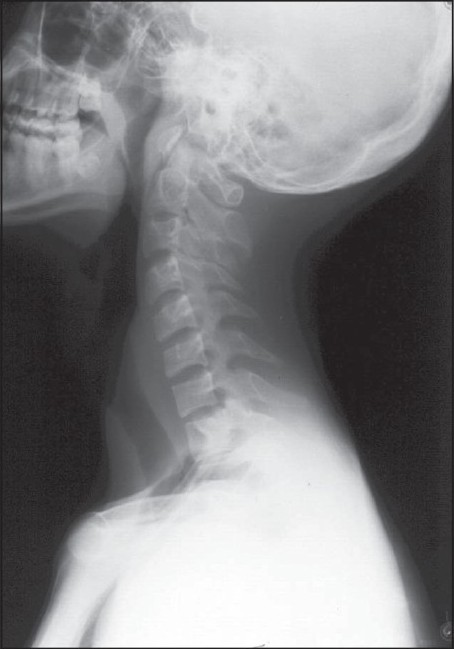
- Plain cervical spine (lateral view) radiograph shows no abnormality and no misalignment of the vertebral bodies
MRI was performed on Magnetom concerto 2002b Siemens 0.2 Tesla MR Unit. The following MR sequences were used:
-
Precontrast axial, sagittal and coronal T1 and T2-weighted spin-echo.
-
Post gadolinium contrast axial and sagittal T1-weighted spin-echo.
-
Axial and sagittal gradient-refocused echo (GRE) flexion cervical MR study.
Cervical MR images in the neutral position showed focal atrophy of the lower cervical cord at the C4-7 vertebral levels but no abnormal intramedullary high signal intensity [Figure 2]. When the neck was flexed, the posterior wall of the cervical dural sac between C4 and D1 vertebral levels was seen to shift anteriorly; the markedly flattened and anteriorly displaced cervical cord was compressed over the posterior surface of the C4 to D1 vertebral bodies. He was diagnosed to have Hirayama's disease (cervical flexion myelopathy). An enhancing epidural space, isointense with the cord on T1-weighted images and hyperintense on T2-weighted images was noted at the posterior aspect of the lower cervical canal [Figures 3 and 4].
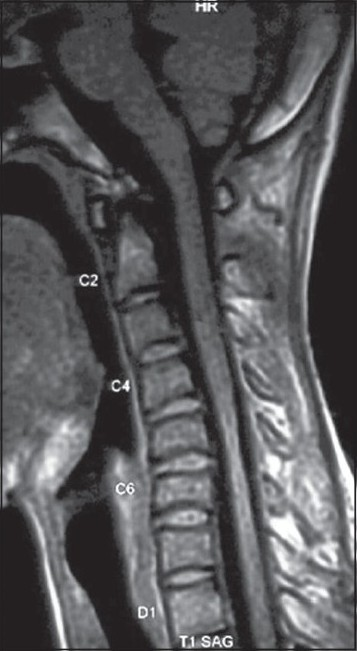
- Cervical T1W-Sagittal MR image in the neutral position shows focal atrophy of the lower cervical cord at the C4-7 vertebral levels but no abnormal intramedullary high signal intensity
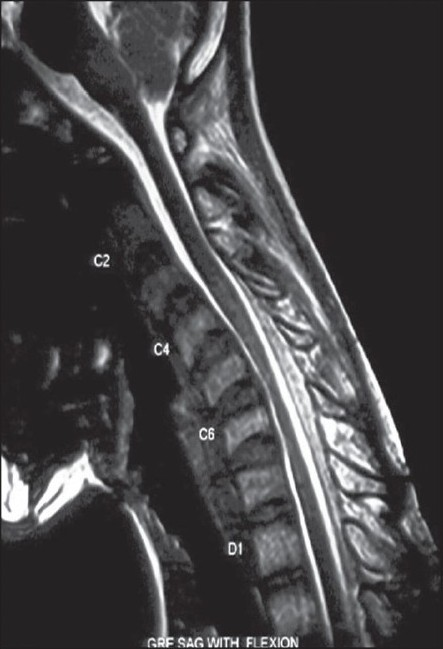
- Cervical GRE-Sagittal MR image in the flexion position shows the posterior wall of the dural sac between C4 and D1 vertebral levels to shift anteriorly, and the anteriorly displaced cervical cord compressed over the posterior surface of the vertebral bodies
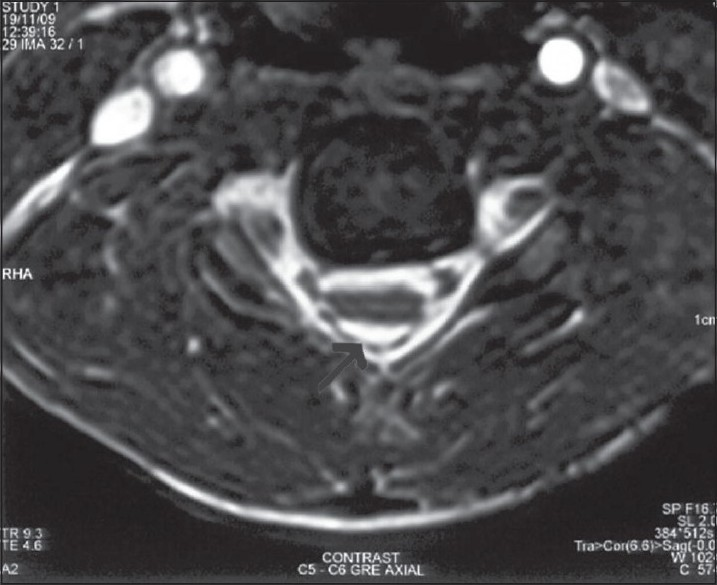
- Cervical GRE-Axial MR image in the flexion position shows the markedly flattened, anteriorly displaced cervical cord due to the epidural lesion (black arrow)
The patient was prescribed a cervical collar to prevent neck flexion.
Discussion
This disease was described first by, and named after, Hirayama in 1959 and most cases of this disease have been reported from Japan and India.[3] This non-familial disorder mostly hits young men, progresses slowly and is often self-limited.
Researchers argue that wasting and weakness associated with this disease is because of imbalanced growth between the patient's vertebral column and spinal canal contents.[4] The imbalanced growth causes a tight dural sac and displaces posterior dural wall anteriorly when the neck is flexed.
Normally, the spinal dura mater is loosely anchored to the vertebral canal by the nerve roots and the periosteum at (a) the foramen magnum and the dorsal surfaces of C2 and C3 and (b) the other at the coccyx.[5] The dura mater is slack enough to adjust to the increased length of cervical spine in flexion. In patients with Hirayama disease, however, the tight dural sac separates the posterior dural sac from its subjacent lamina and on neck flexion, cannot compensate for the increased length of the posterior wall. The posterior dural wall shifts anteriorly, and the cervical spinal cord gets compressed against the posterior margin of adjacent vertebral bodies. This compression affects the anterior spinal artery and causes microcirculatory disturbances in its territory in the lower cervical cord;[1] the anterior horn cells which are vulnerable to ischemia begin to degenerate, resulting in localized cord atrophy of the lower cervical region, weak and wasted hands and forearms.
Hirayama disease is also associated with raised levels of serum IgE and several allergic disorders. Blood often stagnates in the compressed lower cervical cord allowing platelets to aggregate and cause histamine to be released, factors that cause arterial spasm and microcirculatory disturbances.[6] Moreover, the finding that atopic disorders affect young people more than the elderly, men more than women, and Asians more than non-Asians may in part explain the distribution of Hirayama disease.
The key to diagnose this disease during MRI scanning is to obtain images when the neck is flexed. MR studies in flexion not only show the anterior displacement of the posterior wall but also a well-enhanced crescent-shaped lesion in the posterior epidural space of the lower cervical canal.[4] This lesion is thought to represent congestion of the posterior internal vertebral venous plexus.
Although Hirayama disease is a self-limiting disorder, early diagnosis is necessary because a cervical collar, by preventing neck flexion, may arrest the progression of the disorder.[7]
In conclusion, this case showcases how cervical MRI, with neck flexed, can pick up dynamic cord compression and contribute to the diagnosis of Hirayama disease
Source of Support: Nil
Conflict of Interest: None declared.
References
- Non-progressive juvenile spinal muscular atrophy of the distal upper limb (Hirayama's disease) In: De Jong JM, ed. Handbook of Clinical Neurology. Vol 15. Amsterdam: Elsevier; 1991. p. :107-20.
- [Google Scholar]
- Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000;54:1922-6.
- [Google Scholar]
- Juvenile muscular atrophy of unilateral upper extremity; a new clinical entity. Psychiatr Neurol Jpn. 1959;61:2190-7.
- [Google Scholar]
- Magnetic resonance imaging in juvenile asymmetric segmental spinal muscular atrophy. J Neurol Sci. 1997;146:133-8.
- [Google Scholar]
- Anatomy of the human body (37th ed). London, England: Churchill Livingstone; 1989. p. :1086-92.
- Juvenile muscular atrophy of the distal upper limb (Hirayama disease) associated with atopy. J Neurol Neurosurg Psychiatry. 2001;70:798-801.
- [Google Scholar]
- A cervical collar therapy for nonprogressive juvenile spinal muscular atrophy of the distal upper limb (Hirayama's disease) Clin Neurol (Tokyo). 1992;32:1102-6.
- [Google Scholar]






