Translate this page into:
Feasibility and efficacy of thrombolysis in acute ischemic stroke: A study from National Institute of Neurological and Allied Sciences, Kathmandu, Nepal
Address for correspondence: Dr. Lekhjung Thapa, Department of Neurology, National Institute of Neurological and Allied Sciences, Kathmandu, Nepal. E-mail: drlekhjung@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Stroke is the major cause of morbidity and mortality worldwide. The number of stroke patients receiving recombinant tissue plasminogen activator (rt-PA), also known as Alteplase, in the developing world is extremely low. We aim to study the feasibility and efficacy of thrombolysis for the 1st time in our country.
Materials and Methods:
In this retrospective study (July 2012-August 2015), acute ischemic stroke patients who were thrombolyzed within 3 h of stroke onset were included. Their demographic profiles, clinical profiles, risk factors, type of thrombolytic used, and outcomes were systematically recorded and analyzed.
Results:
A total of 9 patients were thrombolyzed. The mean time from the onset of stroke symptoms to first dose of rt-PA (onset to treatment) was 1.2 h. Six patients had good neurological outcome as measured by modified Rankin Scale (mRS). The median mRS at discharge was 3. Thrombolysis-related post treatment complication was noted in 44.4%, of which nonfatal intracranial bleed occurred only in 2 patients (22.2%). None of the patients receiving intravenous tenecteplase had thrombolysis-related complications, and none of the patients had fatal intracranial bleed.
Conclusion:
This study clearly demonstrates the beginning of a feasible and effective thrombolysis in the treatment of acute ischemic stroke in Nepal.
Keywords
Ischemic stroke
Nepal
thrombolysis
tissue plasminogen activator
Introduction
In developing countries, the burden of cerebrovascular diseases has reached epidemic proportions over the past three decades.[1] According to WHO estimates, cerebrovascular disease was the cause of 107.5/100,000 deaths (age-standardized death rate) in Nepal.[2] Provision of recombinant tissue plasminogen activator (rt-PA) therapy in stroke victims within 3 h is an effective treatment with particularly good medium to long-term results. However, it is also known that there is high complication rate with rt-PA treatment versus placebo, particularly with the increased risk of cerebrovascular hemorrhage and an overall mortality of 17%.[3] There is a reasonable amount of literature explaining the difficulties in providing rt-PA treatment in developing countries and the various barriers within these countries that lead to poor provision of thrombolysis.[45] Because of this, provision of effective thrombolysis treatment within developing countries is rare[3] and that which is offered is done so via the private sector.[46] Thrombolysis in Nepal is currently available only in the private sector, primarily because of the great expenditure required to offer it on a public basis and partly because of lack of availability of a dedicated stroke team.
Materials and Methods
In this retrospective study from National Institute of Neurological and Allied Sciences (NINAS), relevant data of all the acute ischemic patients presenting to emergency department from June 2012 to August 2015 was collected. Ethical clearance was taken from the Institutional Review Board. All the patients who presented within 3 h of stroke onset and thrombolyzed were enrolled in our study. As per record, these patients were assessed by our acute stroke team for eligibility for thrombolysis and for appropriate case selection; standardized recommendation for inclusion and exclusion criteria was strictly followed [Table 1].[7] Informed written consents were taken from all the patients or their relatives. All the patients were thoroughly examined and computed tomography (CT) scan brain to rule out hematoma and magnetic resonance imaging stroke protocol to assess early infarct [Figure 1], salvageable tissue, and any evidence of cerebral arterial occlusion was done. Thrombolysis was carried out in Intensive Care Unit (ICU) as per standard protocol [Table 2].[7] Tenecteplase (TNK) was used at a dose of 0.25 mg/kg, administered as a single bolus, with a maximum dose of 25 mg.[8] All the patients’ demographic profiles, clinical profiles, risk factors for stroke, type of thrombolytic used, and outcomes as measured by modified Rankin Scale (mRS) and National Institute of Health Stroke Scale (NIHSS) when available were systematically recorded and analyzed.

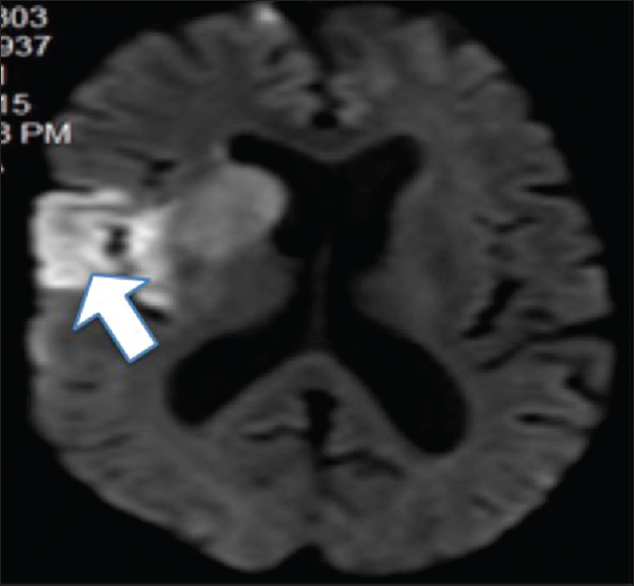
- Diffusion-weighted image showing early right middle cerebral artery infarct (arrow)

Results
A total of 496 patients (317 males and 179 females) of acute stroke had attended emergency room from June 2014 to August 2015, of which 261 had ischemic stroke, 15 had transient ischemic stroke (TIA), 21 had lacunar infarct, 144 had hemorrhagic stroke, and 55 had subarachnoid hemorrhage.
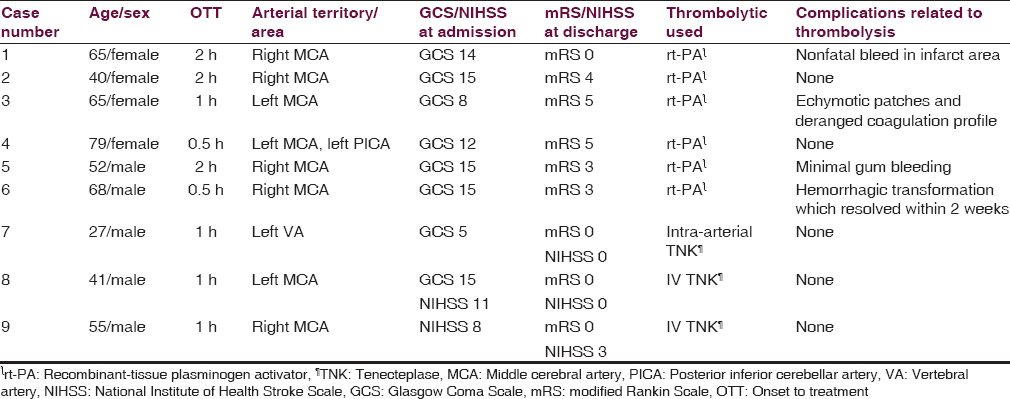
Between 14th June 2012 and August 2015, 11 cases were thrombolyzed, of which 9 cases (3.4%) of acute ischemic stroke attending emergency ward of NINAS were thrombolyzed within 3 h. A total of 6 cases received intravenous (IV) rt-PA, 2 cases received IV TNK, and 1 received intra-arterial TNK.
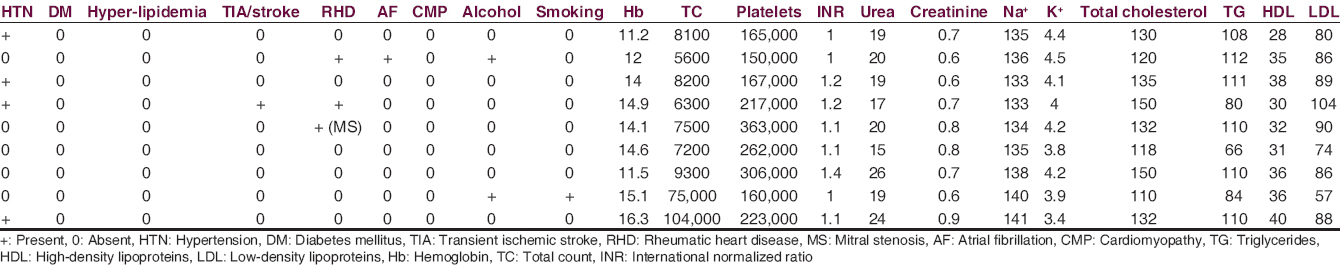
Of 9 patients, 5 were males and 4 were females. Age ranged from 27 to 79 years (mean age 55.3). Among the risk factors, 4 patients (44.4%) had history of hypertension, 1 (11.1%) had previous TIA, 3 (33.3%) had rheumatic heart disease; of which 1 (11.1%) had atrial fibrillation and 1 (11.1%) had undergone commissurotomy for mitral stenosis. Two (22.2%) patients were alcoholic, and 1 (11.1%) patient was a smoker. All of the patients’ biochemical parameters were normal [Table 3].
The mean onset to treatment time was 1.2 h. Thrombolysis-related posttreatment complication was noted in 4 patients (44.4%), of which nonfatal intracranial bleed occurred only in 2 patients (22.2%). None of the patients receiving IV TNK had thrombolysis-related complications, and none of the patients had fatal intracranial bleed. Six patients (66.6%) had good neurological outcome as measured by mRS or NIHSS. The median mRS at discharge was 3 [Table 4].
Discussion
Thrombolysis is one of the proven potential treatments for the management of acute ischemic stroke.[910] For the 1st time in our country, we thrombolyzed 9 cases of acute ischemic stroke presenting within the window period of 3 h. Eight of our cases received IV thrombolysis while intra-arterial thrombolysis was done in 1 case of vertebrobasilar stroke. Stroke is known to be the leading cause of disability and mortality worldwide (5.7 million annual deaths).[10] Although we get 8–10 cases of acute ischemic stroke per week at our institution, most of them reach us late and do not qualify for thrombolysis. Prehospital delay, financial constraints, and lack of infrastructure are main barriers of thrombolytic therapy.[4] In Nepal, all the three problems including stroke awareness, transportation facility, and difficulty in easy access due to geographical factors do exist. To offer the best benefit to the patients, we have thrombolyzed patients beyond 3 h and have currently extended therapeutic window in our protocol up to 4.5 h. The mean age of patients we thrombolyzed was 55.3. Low- and middle-income countries like ours, where the average age of patients with stroke is 15 years younger, have high stroke burden.[6] We used rt-PA in most of the cases, and it is the only medically approved biological treatment for acute ischemic stroke for use within 4.5 h of stroke onset and is recommended by most national and international stroke associations as a first-line therapy.[1112] After the result of NINDS trial, rt-PA has been widely used in United States and Europe.[13] The safety and effectiveness of rt-PA have been proven at an IV dose of 0.9 mg/kg (10% bolus during 1 min and 90% infusion over 1 h) within 3 h in the United States and within 4.5 h of stroke onset in Europe.[1214] Although Japan Alteplase Clinical Trial study suggests a dose of 0.6 mg/kg for clinical efficacy and safety of Japanese patients,[15] we used the dose recommended by Food and Drug Administration (FDA) and all of our patients receiving rt-PA received FDA-approved dose protocol.[7] TNK, a newer thrombolytic agent, has been approved for the treatment of acute myocardial infarction and has given hope for the possibility of a much safer alternatives in acute ischemic stroke.[8131617] Our initial encouraging results with rt-PA prompted us to use a more safer agent TNK because of mainly two reasons: (1) rt-PA was not imported by the pharmaceuticals because it was not used in the country as per their expectations and (2) TNK is widely used by cardiologists, easily available, and researches have proven it to be safer, effective, and easy to use in acute ischemic stroke patients.[8131617] We had counseled both the patients and family members before administering TNK about their disease, therapeutic options, unavailability of rt-PA and the trial stage of the TNK, their results, and pending FDA approval for use in stroke. Interestingly, all three patients (case 7, 8, and 9) receiving TNK had an excellent recovery without any complications and was discharged with mRS 0 and a favorable NIHSS [Table 4], which proves it to be safe and effective in our setting. Although this has to be confirmed with a large-scale study, it has encouraged us to use TNK in acute ischemic stroke cases in days to come.
We had administered thrombolytic agents in ICU set-up after ruling out all the contraindications[13] by our acute stroke team. These patients were closely monitored, and we did come across a few nonfatal complications [Table 4]. A prospective, open, multicenter, multinational, observational study SITS-ISTR (Safe Implementation of Treatments in Stroke-International Stroke Thrombolysis Registry) for 700 clinical centers practicing thrombolysis for acute stroke in 35 countries demonstrated the rate of symptomatic intracerebral hemorrhage of 1.6% at 3 h and 2.2% within 3–4.5 h; mortality rate of 12.2% at 3 h and 12.7% within 3–4.5 h, and functional independence of 56.3% at 3 h and 58.0% within 3–4.5 h.[1218] High percentage (22.2%) of nonfatal intracranial bleed (only in rt-PA group) in our series could be because of less number of cases and also the bleeding phenomena could just be a part of hemorrhagic conversion as it happens in other cases of acute ischemic stroke. To note, these patients were aged 65 years and 68 years and first patient had a history of hypertension. Age, hypertension, diabetes mellitus, cerebral amyloid angiopathy, early ischemic signs CT, and volume of cerebral ischemic lesions on diffusion-weighted image are known potential factors related to rt-PA-related intracranial hemorrhage.[19]
Despite the fact that thrombolysis using rt-PA has a higher potential for a better outcome, it is underused in patients with acute ischemic stroke. The low rates of rt-PA use in acute stroke are lower patient education and perception of risk by physicians.[20] Studies have shown that physicians would agree to administer rt-PA in such patients after a formal consultation with a stroke neurologist.[21] However, we do not have such studies from our country although there are several hospital-based stroke studies done in Nepal and majority of them have studied risk factors, and most of them are descriptive.[22]
Nepal has not been able to evidently introduce IV rt-PA which has been approved for more than a decade ago in developed countries. There are many challenges such as availability of trained personnel that includes stroke team primarily for the treatment of acute ischemic stroke and for using rt-PA. The number of clinical neuroscientists in Nepal is very less; however, the burden of stroke is increasing day-by-day. The NINAS consists of a trained stroke team including neurologists, neuroradiologists, and neurosurgeons working around the clock, and hence thrombolysis can be easily performed within a defined period. However, rt-PA is not easily available in all the hospitals of Nepal and is a costly medicine (NPR 33,000/-[approximately 330 US dollars]), which might not be affordable for the general Nepalese population. Nevertheless, our report demonstrates and brings hope for the beginning of effective thrombolysis in the treatment of acute ischemic stroke in Nepal.
Conclusion
Although our study has all the inherent limitations of a retrospective study, our report clearly demonstrates the beginning of a feasible and effective thrombolysis in the management of acute ischemic stroke in Nepal. This study indicates a landmark achievement for the country and will hopefully initiate the spread of wider reaching thrombolytic therapy for acute ischemic stroke across Nepal and developing countries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank all the registrars, medical officers, and staff of NINAS for helping in timely patient management and data collection. We would also like to thank Mr. Shakti Shrestha and Mr. Ramesh Sharma Poudel for manuscript preparation. We thank Ms. Jana Hermann and Ms. Helen Jessie Uriah for English editing.
References
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med. 1995;14:1581-8.
- [Google Scholar]
- Barriers of thrombolysis therapy in developing countries. Stroke Res Treat 2011 2011:686797.
- [Google Scholar]
- Treatment of acute stroke: Is there a role for streptokinase when is not available? J Postgrad Med Inst. 2013;27:122-8.
- [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
- [Google Scholar]
- A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099-107.
- [Google Scholar]
- Intercollegiate Stroke Working Party (ISWP). National Clinical Guidelines for Stroke. (2nd ed). Suffolk: The Lavenham Press Ltd; 2010.
- [Google Scholar]
- Management of ischaemic stroke in the acute setting: Review of the current status. Cardiovasc J Afr. 2013;24:86-92.
- [Google Scholar]
- Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): An observational study. Lancet. 2007;369:275-82.
- [Google Scholar]
- UK drug agency announces review of use of alteplase after stroke. BMJ. 2014;349:g5355.
- [Google Scholar]
- Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT) Stroke. 2006;37:1810-5.
- [Google Scholar]
- Scottish Intercollegiate Guidelines Network. Management of Patients with Stroke or TIA: Assessment, Investigation, Immediate Management and Secondary Prevention, a National Clinical Guideline. Number 108. Edinburgh: SIGN; 2008.
- [Google Scholar]
- Thrombolysis: Newer thrombolytic agents and their role in clinical medicine. Heart. 2003;89:1358-62.
- [Google Scholar]
- Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): An observational study. Lancet. 2008;372:1303-9.
- [Google Scholar]
- Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative acute stroke study. Stroke. 1997;28:957-60.
- [Google Scholar]
- Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol. 2006;63:661-4.
- [Google Scholar]
- Views of emergency physicians on thrombolysis for acute ischemic stroke. J Brain Dis. 2009;1:29-37.
- [Google Scholar]






