Translate this page into:
Secondary surgical epilepsy as a predictive recurrence factor in patients with grade I meningioma: An observational clinical study
*Corresponding author: Daniele Armocida, Department of Human Neurosciences, Division of Neurosurgery, Sapienza University, Policlinico Umberto I Sapienza University, Rome, Italy. danielearmocida@yahoo.it
-
Received: ,
Accepted: ,
How to cite this article: Armocida D, Zancana G, Arcidiacono UA, Corvino S, Pesce A, Paolini S, et al. Secondary surgical epilepsy as a predictive recurrence factor in patients with grade I meningioma: An observational clinical study. J Neurosci Rural Pract. 2025;16:22-9. doi: 10.25259/JNRP_37_2024
Abstract
Objectives
The World Health Organization (WHO) grade I intracranial meningiomas (IMs) maintain a certain risk of recurrence (~10%) even if a gross total resection is achieved. Most studies analyzing predictive factors of benign meningioma recurrence focus on histological changes and factors related to radiologic-therapeutic follow-up. Few authors have speculated on the role of meningioma-related epilepsy on the risk of developing recurrence. The presence of seizures and the factors predictive of their onset have remained relatively understudied to date in meningioma patients.
Materials and Methods
In this retrospective observational analysis, we studied clinical, radiological, and biological factors in 291 grade I IMs. In multivariate analysis of radiological and clinical variables, we evaluated the outcome and the risk of recurrence. A special focus was given to the presence of seizures: We reported patients who had seizures at onset and compared them with patients who experienced seizures in the postoperative phase and who developed secondary epilepsy after surgery.
Results
We found that grade I IMs who developed a secondary form of epilepsy after surgery have a significant correlation with the presence of recurrence diagnosed during the follow-up (17/50 patients with seizures 34%, P = 0.02). Yet, IM patients who debuted with seizures do not have a significant risk of developing recurrence during follow-up. We recognize that there is a significant difference in the expression of ki67% (t = −2.03; df = 105; P = 0.04) between the group that showed recurrence (M = 8.79) and the one that never had recurrence (M = 5.14).
Conclusion
Our analysis suggests the role of post-operative epilepsy as an independent prognostic factor, not correlated with pre-operative seizure on meningioma recurrence. We confirmed that a significant cell replication factor such as ki67 significantly correlates with the risk of recurrence and probably indirectly correlates with the risk of developing post-operative epilepsy.
Keywords
Meningioma recurrence
Meningioma surgery
Meningioma
Post-operative complications
Post-operative seizure
INTRODUCTION
Intracranial meningiomas (IMs) are the most common non-malignant head tumors in adults.[1] The World Health Organization (WHO) classification distinguishes three histological grades (1–3) and 15 subtypes[2] without any update in the WHO 2021. Although most IMs are histologically benign, recurrence is common, even for the WHO type I meningiomas.[3,4]
Meningioma recurrence is one of the most critical factors directly impacting the patient’s outcome,[5] whereas histology[6] and the extent of resection (EOR) (measured using the Simpson grade)[7] are the main responsible.[8] Other supposed predictors of post-operative recurrence are tumor location, size, invasion, peritumoral brain edema (PBE), age, and gender.[9] Some studies[3,4,10-12] analyze other predictive factors of benign meningioma recurrence, focusing on histological changes and factors related to radiologic-therapeutic follow-up.[13]
However, few clinical elements have been analyzed as risk factors, considering that most modern studies consider national cancer databases and registries (e.g., Surveillance, Epidemiology, and End Results in the United States) without having the opportunity to study cases treated at their referral centers and thus having all the anamnestic and clinical outcome data. Some patients develop seizures resulting from tumor growth. Seizures are, for some, a presenting symptom, while in others, the seizures develop after surgical removal of the tumor. It is assumed that the pathophysiology behind seizures is a combination of the tumor’s mass effect on the epileptogenic cortex, disturbances of neurotransmitter pathways, and/or acid/base derangements from cerebral edema.
The presence of seizures and the factors predictive of their onset, especially their control, have remained relatively understudied to date[14,15] in meningioma patients. Several times over the years, some authors have speculated on the possible role of meningioma-related epilepsy on the risk of developing recurrence.[16-18] In this retrospective observational analysis, we studied clinical, radiological, and biological factors in benign meningiomas (WHO grade I) to investigate the possible role of seizures in the risk of recurrence.
MATERIALS AND METHODS
We performed a retrospective observational study of a consecutive surgical series of patients suffering from benign IMs operated between January 2016 and December 2020. Our institutional review board approved this study’s method, which included ethical considerations. Informed consent was obtained from all participants. Our study included 340 patients diagnosed with IMs. We documented various patient characteristics at the time of diagnosis, including age, gender, duration of hospital stay and follow-up, initial symptoms, tobacco use, existing health conditions (with emphasis on heart disease and high blood pressure), and functional status (assessed with Karnofsky performance scale). In addition, we recorded results from neurological and clinical examinations, as well as the occurrence of seizures at onset, which were confirmed through electroencephalography (EEG).
Regarding the final histological diagnoses, we selected WHO type I IM reporting the histological subtypes and proliferation index measured with immunohistochemistry with Ki67%. For the radiological assessment, we documented various parameters including the intracranial position of the lesion (noting the affected side and primary lobe), whether the subtentorial compartment was affected, the maximum tumor diameter (in cm), and tumor volume (in cm3) utilizing isotropic volumetric contrast-enhanced T1-weighted sequences before and after intravenous injection of the paramagnetic contrast agent (gadolinium). T2-weighted and fluid-attenuated inversion recovery sequences were employed to determine edema volumes (in cm3 before antiedemigen therapy). The volume of the contrast-enhancing lesion and PBE was determined by outlining a region of interest in a volumetric enhancing post-contrast study weighted in T1 (a multivoxel study) and T2, conforming to the boundaries of the contrast-enhancing lesion using the Horos software,[9] in accordance with our previously established institutional protocol for IM.
Our institution’s digital database obtained clinical and radiological information, whereas telephone interviews were used to obtain outcome data.
Our follow-up plan necessitated that each patient receive a contrast-enhanced magnetic resonance imaging (MRI) within 72 hours after the procedure, which utilized Simpson’s grade to confirm the extent of surgical resection and identify patients with a gross total resection (GTR). Following this, patients underwent clinical (with performance status evaluation) and MRI imaging evaluations at 1 month, 6 months, and 1 year. If recurrence was detected, the patient was reevaluated for a second surgical procedure or considered for a stereotactic radiosurgery protocol. Our study documented the occurrence of complications, disease recurrence, and the subsequent requirement for additional interventions.
Specific focus was given to the occurrence of seizures. Initially, we documented patients who had seizures at the beginning. We conducted a group analysis comparing patients who experienced seizures in the late post-operative period (only including those patients who had multiple seizures 1 week after surgery, Group A) with patients who did not have seizures and did not require antiepileptic drugs (AEDs, Group B). Epilepsy was defined as a “characteristic cluster of clinical and electroencephalographic features, often supported by other specific etiological findings” following the international league against epilepsy (ILAE) definition;[19] after performing EEG and neurological evaluation, it was submitted to chronic therapy with AEDs.
Statistical methods
The sample was analyzed using the Statistical Package for the Social Sciences version 25. We conducted univariate and multivariate analyses to examine the relationship between the factors and brain edema. Comparisons between nominal variables were made with the Chi-squared test. We used the one-way and multivariate analysis of variance analysis, contrast analysis, and post hoc tests to compare the mean of the EOR, which was measured with Simpson Grade. Pearson’s bivariate correlation was used to investigate the correlations between continuous variables. The threshold for statistical significance was set at P < 0.05.
RESULTS
The study’s final analysis included 291 IMs WHO type I. Following the application of inclusion-exclusion criteria, 37 individuals were omitted due to lack of follow-up imaging, 10 were excluded because of loss to follow-up, and 2 were removed for not providing informed consent for scientific data processing.
The resulting cohort comprised 101 males (34.7%) and 190 females (65.3%), with a mean age of 60.38 ± 13.56 years, ranging from 20 to 90 years old. Details on patient demographics and clinical data are shown in Table 1. Our cohort’s demographics, hospitalization time, and follow-up were consistent with previously reported extensive studies.[20-22]
| Patients | No. 291 | |
|---|---|---|
| Age | Mean: 60.38 Median: 62 Sd: 13.56 | Min: 20 Max: 90 |
| Sex (Female) | F: 190–65.3% M: 101–34.7% | |
| Smoke | 98=33.7% | |
| Hypertension | 108=37.1% | |
| Clinical Debut | Incidental (1)=42–14.4% | Headache (4)=34–11.7% |
| Dizziness (2)=32–10.9% | Seizure (5)=88–30.2% | |
| Focal deficit (3)=60–20.6% | Mental alteration (6)=35–12% | |
| Hospitalization (330 pts) | Mean: 17.76 Median: 13 Sd: 17.23 | Min: 5 Max: 209 |
| Follow-up (months) | Mean: 47.76 Median: 47 Sd: 14.82 | Min: 12 Max: 72 |
| Hystological type | 1=meningothelial-205–60.3% | 6=Fibroblastic-13–4.4% |
| 2=Psammomatose-16–4.7% | 7=Secretory-12–3.5% | |
| 3=Transitional-22–6.5% | 8=Angiomatous-9–2.6% | |
| 4=Microcystic-8–2.4% | 9=Lymphoplasmacitic-1–0.3% | |
| 5=Fibrous - 13-3.8% | 10=Metaplastic-5–1.5% | |
| Biological type switch | 3/291–1% | |
| Location/position | 1=clinoid-11–3.8% | 8=occipital convexity-12–4.12% |
| 2=APC-12–4.1% | 9=sphenoid wing-20–6.87% | |
| 3=falx-39–13.4% | 10=tuberculum sellae-9–3.1% | |
| 4=parasagittal parietal-19–6.53% | 11=planum sphenoidal-8–2.75% | |
| 5=parasagittal frontal-26–8.9% | 12=subtentorial-21–7.22% | |
| 6=olfactory groove-14–4.8% | 13=temporal convexity-15–5.15% | |
| 7=frontal convexity-85–29.2% | 14=sphenopetroclival-12–4.12% | |
| Gross total resection (Simpson Grade I) | 253=87.4% | |
| Ki67/MIB-1 | Mean | 9 |
| DS | 8 | |
| Epilespy after surgery | 41=14.1% | |
| Seizure therapy | 88=30.2% | |
| Recurrence | 33=11.3% | |
The most frequent clinical onset leading to the IMs’ radiological diagnosis was a seizure in 88 patients (30.2%), followed by the onset of focal motor or sensory deficits in 60 patients (20.6%), incidental finding following imaging performed for other reasons in 42 patients (14.4%), mental status alteration or mood disturbance in 35 patients (12%), headache in 34 patients (11.7%), and dizziness and alterations in walking in 32 patients (10.9%). Every patient who experienced a seizure was treated with (AEDs, levetiracetam in 77.6% of cases). Recurrence with radiological evidence of meningioma regrowth was found during follow-up in 33 patients (11.3% of the population).
We analyzed the histological type of WHO grade I meningiomas and its location site in our series: We show that histological type and site do not significantly (both P = 1) influence the risk of recurrence.
Our recent study[20] on the same patient series reaffirmed that neurological function recovery and performance grade post-surgery remained significantly lower in relation to age, both immediately after the operation and during the final follow-up assessment (both P < 0.01). Multivariate analysis revealed a correlation between increasing age and higher ki67 values and mitotic index. However, these factors were not associated with edema volume, recurrence rate, Simpson grade, or survival (P = 1).
The grade I meningothelial form was the most common histological subtype. Cluster analysis examined the relationship between histologic type and recurrence risk but did not identify any specific histotype as having a higher likelihood of recurrence during follow-up.
Regarding surgical radicality, 253 patients (87.4%) achieved a Simpson type I grade resection, as evaluated by the initial post-operative MRI. This finding logically corresponds to a non-significant difference in recurrence rate (P = 0.598) and clinically to the progression of the lesion to a higher grade and/or malignancy (P = 0.59).
We analyzed immunohistochemical data, particularly ki67, as the main index of cell replication and a known risk factor for recurrence risk. We also recognize in our series that there is a statistically significant difference in the expression of ki67% (t = −2.03; df = 105; P = 0.04) between the group that showed recurrence (M = 8.79) and the one that never had recurrence (M = 5.14, [Figure 1]). In contrast, we did not show a statistically significant difference (t = −0.23; df = 72; P = 0.818) between the group that had post-operative epilepsy (M = 6) and the group that did not (M = 5.52).
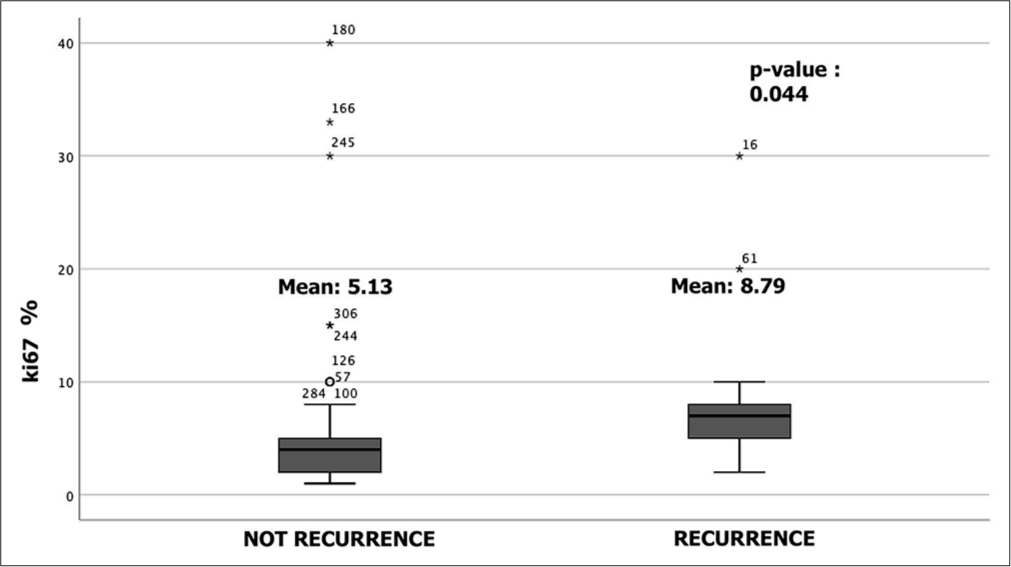
- Box-plot of analysis of ki67% immunohistochemical data (t-student test). We also recognize a significant difference in the expression of ki67 (t = −2.034; df = 105; P = 0.044) between the group that showed recurrence (M = 8.79) and the one that never had recurrence. °: Isolated results, *: Outliners of data
Group analysis
The study analyzed the onset of drug-dependent epilepsy in the post-surgical follow-up phases by examining clinical, radiological, and surgical variables [Table 2]. No correlation was found between radiological and clinical variables, including age, sex, hypertension, cigarette smoking, tumor diameter, volume, edema, multiple lesions, progesterone expression, ki67%, subtentorial lesion, and presence of complications, and the presence of post-surgical epilepsy. Patients with seizures at the time of surgery were found to be at the highest risk of developing epilepsy after surgery (17/50 patients, 34%, P = 0.02, [Figure 2]). However, patients with meningioma who developed seizures did not have a significant risk of developing recurrence during follow-up [Figure 3a]. Among IM patients who developed pharmacologically treated epilepsy after surgery, there was a significant correlation with the presence of recurrence [Figure 3b]. Interestingly, there was no statistically significant correlation between the risk of recurrence and the administration of anti-epileptic drugs (21 patients, P = 0.49).
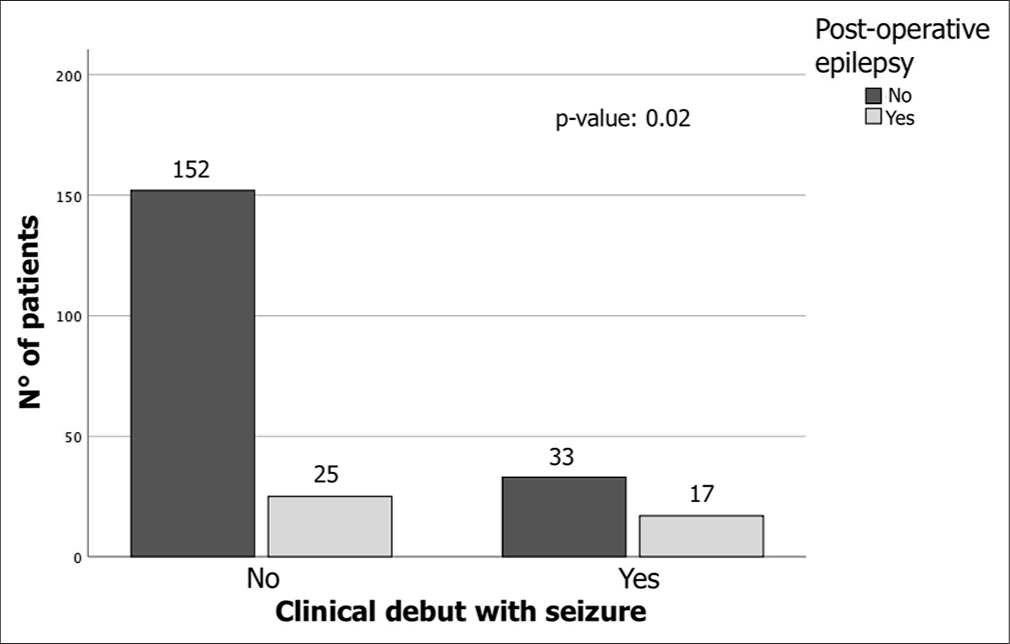
- Chi-square analysis shows that meningioma patients who clinically debut with seizures are most frequently associated with an elevated risk of developing epilepsy even in the post-operative phase (17/50 patients with seizures 34%, P = 0.02).
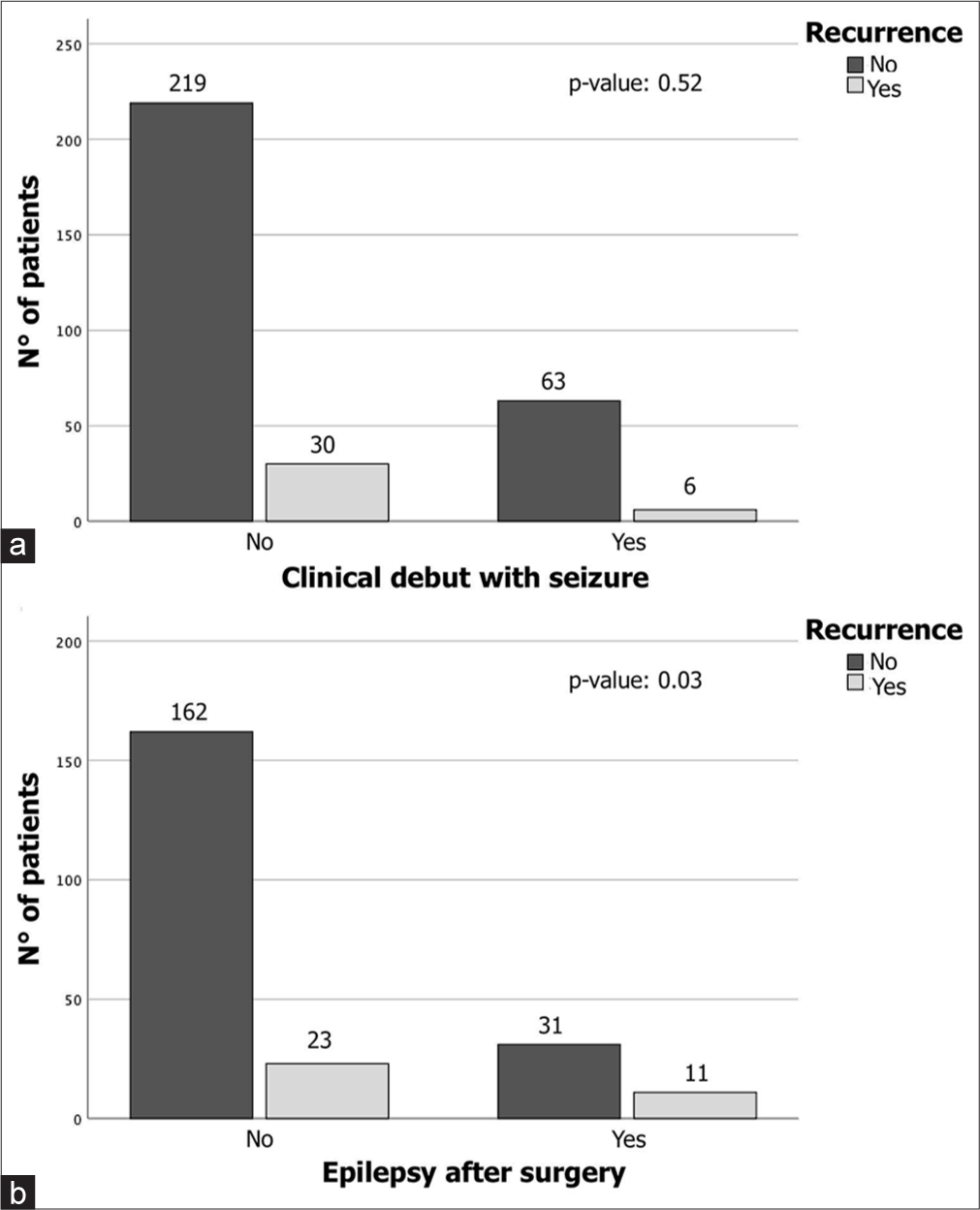
- Chi-square analysis shows a significant difference between (a) meningioma patients who debuted with seizures that do not have a significant risk of developing recurrence during follow-up and (b) patients who developed a pharmacologically treated form of epilepsy after surgery (P < 0.01).
| Group A | Group B | P-value | |
|---|---|---|---|
| Epilepsy after surgery (50 patients) | Seizure-free (241 patients) | ||
| Female gender | 30 | 134 | 0.537 |
| Older age (>65) | 20 | 82 | 0.402 |
| Smoke habit | 12 | 43 | 0.543 |
| Hypertension | 11 | 54 | 0.156 |
| Multiple | 5 | 5 | 1 |
| Subtentorial | 2 | 20 | 0.183 |
| Progesteron+ | 6 | 25 | 0.577 |
| Ki67% (mean) | 6 | 5.52 | 0.818 |
| Complications after surgery | 10 | 28 | 0.127 |
| Bleeding after surgery | 1 | 4 | 0.7 |
| Ischemia after surgery | 1 | 5 | 0.695 |
| Surgical site infections | 3 | 14 | 0.616 |
| Seizure symptoms at diagnosis | 17 | 33 | 0.002 |
| Major diameter (mean, cm) | 4.64 | 4.21 | 0.156 |
| Lesion volume (mean, cm3) | 39.91 | 34.86 | 0.442 |
| Edema volume (mean, cm3) | 27.3 | 31.96 | 0.644 |
DISCUSSION
The WHO type I meningiomas have a typically low risk of recurrence (~10%) if a GTR is performed.[6,7,13,23,24] We confirm an overall recurrence rate of 11.3% (33 patients) within a median of 22.4 months, whereas 87.4% (253 patients) of meningioma patients underwent Simpson grade 1 excision.
With this study, we report two identified clinical and histological predictive factors of recurrence.
First, we report that the onset of post-operative epilepsy can be considered a predictive factor of tumor recurrence. In this series, Grade I meningioma patients who developed a secondary form of epilepsy after surgery have a significant correlation with the presence of recurrence diagnosed during the follow-up.
This is not the first time a study has hypothesized a correlation between post-operative epilepsy and the onset of recurrence: A study by Zheng et al. reported that 66.7% of those who developed tumor progression and recurrence experienced late post-operative seizures.[23] Xue et al.[13] reported that 14.1% of patients with late post-operative seizures developed recurrence, identifying a trend for higher seizure frequency in conjunction with signs of tumor recurrence.
Among several post-operative symptoms that may occur, seizures are recognized in 30–40% of meningioma patients.[4,25] Most cases of seizures in patients with meningioma are related to a positive pre-operative history of seizures.[11,26] In 10–15% of patients with meningioma without a history of epilepsy,[27-30] seizures may occur postoperatively. Several factors have been hypothesized to cause post-operative seizures, including tumor location, degree of infiltration, size, and edema. However, our analysis did not reveal any significant relationships, and it was not possible to demonstrate the degree of meningioma adhesion to the brain parenchyma in a retrospective analysis.
We identified how a patient with a history of pre-operative seizures shows an increased risk of developing post-operative epilepsy but does not show a risk of developing recurrence per se.
Second, analyzing the biological variables, we found an interesting correlation with the expression of Ki67. The Ki67 protein is found in cells undergoing division, and its quantity strongly correlates with tissue proliferation rates.[31,32] As a good indicator for identifying the proliferative fraction of a cell population,[33] Ki-67 has been shown in numerous studies to have a significant association between increased labeling index and tumor recurrence.[27,32,33]
In meningiomas, the Ki-67 labeling index rises in conjunction with the WHO grade. Notably, recurring WHO grade I tumors exhibit a higher Ki-67 labeling index.[14,34-36] It is important to note that the significant cell replication factor such as ki67 significantly correlates with the risk of recurrence, as already reported in the literature, and probably indirectly correlates with the risk of developing postoperative epilepsy.
The reason for this clinical-pathological association may be twofold. Suppose a patient develops secondary epilepsy postoperatively, in that case, it may mean that there may be a tumor active micro-residue that is not shown on the MRI performed within 72 hours after surgery but still causes cortical irritation. This aspect is well known in other intracranial tumors, whereas freedom from seizures after surgery is predicted by GTR.[4] Further, seizures inevitably leading to a pathophysiological state of post-critical edema may promote residual tumor cells’ rooting and biological activity (measurable indirectly with ki67%),[37,38] leaving the patient less prone to but not completely free of seizures after the tumor resection.[27] The disease recurrence, starting from a proliferative cellular level, can stimulate the onset of new PBE, stimulating the epileptogenic region. In the multivariate analysis, we studied the other variables that may be concomitant with the development of epilepsy, high ki67%, and the risk of recurrence; among them, we analyzed the impact of tumor size, PBE, and site, but we did not find a significant correlation.
Most of the studies indicate that the majority of post-surgery seizures occur within the first week following the operation, with less than one-third of patients developing a secondary form of epilepsy.[39] Various factors can contribute to the onset of post-operative seizures, particularly in patients who have not previously experienced seizures. These factors include strong adhesions encountered during surgery, the necessity for microdissection, and potential damage or irritation to the cortical surface.[40] In cases involving skull base lesions, the need for retraction and manipulation to achieve complete resection can result in additional cortical damage and swelling.[25] This process is further exacerbated by increased cellular proliferation in the affected area.
Further studies and limitations
One of the main limitations of this study was that it was conducted retrospectively, which made it impossible to effectively conduct a risk assessment through randomization. Further, part of the analysis and follow-up protocol was achieved during the COVID-19 pandemic with its limitations.[41]
Apart from the biological markers mentioned earlier, other important molecular factors have also been identified in meningiomas. The epithelial membrane antigen and progesterone receptor (PR) are frequently used diagnostic markers for these tumors.[18,31] Moreover, recent research has confirmed a link between deoxyribonucleic acid methylation in meningiomas and the risk of recurrence. Our study did not examine the role of AED therapy in pre-operative seizures or preventing the development of seizures. Different studies have reported no significant differences in the incidence of early seizures and prophylactic AED therapy.[42,43] Yang et al.[9] showed that prophylactic use of AEDs did not impact the seizure rate for several reasons. They suggest that surgery is effective in removing the tumor without damaging surrounding brain tissue because of the benign origin of the neoplasm and its most intact and well-defined border. As a result, the likelihood of a seizure attack after surgery is relatively low. Seizure attacks can be reduced by controlling relevant factors, and we strongly agree with this assumption.
CONCLUSION
With this retrospective study, the authors suggest a possible role of epilepsy as an independent prognostic factor, not correlated with pre-operative seizure, on early benign meningioma recurrence. Seizures are a relatively common presentation in meningioma patients. They are likely to have tumors with a higher grade, atypical histology, elevated Ki-67 expression, and brain invasion. Although there is no correlation with the onset of seizure, the post-operative onset of epilepsy in post-operative time has a positive correlation with tumor recurrence.
Authors’ contributions
AF and DA: Conceptualization. AP and UAA: Methodology. SP: Validation. DA, UAA, GZ: Investigation. AF: Research. AF, BA, AP: Data collection. DA and UAA: Writing the first manuscript draft. SC: Writing review and editing. AF, RB: Visualization and supervision. AF and AS: Project administration.
Ethical approval
The study is approved by the Institutional Review Board of Policlinico Umberto I, Rome, Italy, (IRB 6168 Prot. 0935/2020). This study adheres to the ethical guidelines for human medical research as outlined in the Helsinki Declaration.
Declaration of participant consent
The authors certify that they have obtained all appropriate participant consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: This work was supported by the Italian Ministry of Health (Current Research 2023) and the University Sapienza of Rome (Italy).
References
- The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23:1231-51.
- [CrossRef] [PubMed] [Google Scholar]
- Brain invasion and the risk of seizures in patients with meningioma. J Neurosurg. 2018;130:789-96.
- [CrossRef] [PubMed] [Google Scholar]
- Abundant immunohisto-chemical expression of dopamine D2 receptor and p53 protein in meningiomas: Follow-up, relation to gender, age, tumor grade, and recurrence. Brazilian J Med Biol Res. 2015;48:415-9.
- [CrossRef] [PubMed] [Google Scholar]
- Seizure control for patients undergoing meningioma surgery. World Neurosurg. 2013;79:515-24.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life after surgery for intracranial meningioma. Cancer. 2018;124:161-6.
- [CrossRef] [PubMed] [Google Scholar]
- Peritumoral brain edema in relation to tumor size is a variable. That influences the risk of recurrence in intracranial meningiomas. Tomography. 2022;8:1987-96.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative 3D volume reconstruction of the posterior wall of the sphenoid sinus with Horos: A free, simple and reliable tool in endoscopic endonasal trans-sphenoidal surgery. Neurocirugia (Astur: Engl Ed). 2022;33:219-26.
- [CrossRef] [Google Scholar]
- Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88:831-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylactic AEDs treatment for patients with supratentorial meningioma does not reduce the rate of perioperative seizures: A retrospective single-center cohort study. Front Oncol. 2020;10:568369.
- [CrossRef] [PubMed] [Google Scholar]
- European health systems and cancer care. Ann Oncol. 2003;14(Suppl 5):v41-60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical management of supratentorial non-skull base meningiomas. Cancers (Basel). 2022;14:5887.
- [CrossRef] [PubMed] [Google Scholar]
- Late outcome of operations for supratentorial convexity meningiomas. Report on 207 cases. Surg Neurol. 1984;22:588-94.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term control and predictors of seizures in intracranial meningioma surgery: A population-based study. Acta Neurochir (Wien). 2018;160:589-96.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of seizures before and after neurosurgical treatment of intracranial meningiomas. Clin Neurol Neurosurg. 2018;165:60-6.
- [CrossRef] [PubMed] [Google Scholar]
- Topographic distribution of intracranial meningioma's recurrences: Localized versus diffuse-multicentric. London: IntechOpen; 2020
- [CrossRef] [Google Scholar]
- Thecombination of mitotic and Ki-67 indices as a useful method for predicting short-term recurrence of meningiomas. Surg Neurol. 2004;61:149-55.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between histological grade and MIB-1 and p53 immunoreactivity in meningiomas. Clin Neuropathol. 2005;24:219-24.
- [Google Scholar]
- Methodology for classification and definition of epilepsy syndromes with list of syndromes: Report of the ILAE task force on nosology and definitions. Epilepsia. 2022;63:1333-48.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial meningioma in elderly patients. Retrospective multicentric risk and surgical factors study of morbidity and mortality. Diagnostics (Basel, Switzerland). 2022;12:351.
- [CrossRef] [PubMed] [Google Scholar]
- Epilepsy and driving: Potential impact of transient impaired consciousness. Epilepsy Behav. 2014;30:50-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mood disorders in patients with epilepsy: Epidemiology and management. CNS Drugs. 2002;16:369-78.
- [CrossRef] [PubMed] [Google Scholar]
- Early and late postoperative seizure outcome in 97 patients with supratentorial meningioma and preoperative seizures: A retrospective study. J Neurooncol. 2013;114:101-9.
- [CrossRef] [PubMed] [Google Scholar]
- Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg. 2019;133:1345-54.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of developing postoperative deficits based on tumor location after surgical resection of an intracranial meningioma. J Neurol Surg B Skull Base. 2019;80:59-66.
- [CrossRef] [PubMed] [Google Scholar]
- The role of prophylactic antiepileptic drugs for seizure prophylaxis in meningioma surgery: A systematic review. J Clin Neurosci. 2017;43:47-53.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting outcome of epilepsy after meningioma resection. Neuro Oncol. 2016;18:1002-10.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors analysis and a nomogram model establishment for late post-operative seizures in patients with meningioma. J Clin Neurosci. 2020;80:310-7.
- [CrossRef] [PubMed] [Google Scholar]
- Management of atypical and anaplastic meningiomas. Neurosurg Clin N Am. 2016;27:239-47.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrences in meningioma after surgery. Acta Neurochir (Wien). 1989;100:104-7.
- [CrossRef] [PubMed] [Google Scholar]
- The significance of immunohistochemical expression of Ki-67, p53, p21, and p16 in meningiomas tissue arrays. Pathol Res Pract. 2008;204:305-14.
- [CrossRef] [PubMed] [Google Scholar]
- Biology of meningiomas. Acta Neurochir (Wien). 2000;142:493-505.
- [CrossRef] [PubMed] [Google Scholar]
- Four independent predictors of post-operative seizures after meningioma surgery: A meta-analysis. World Neurosurg. 2019;130:537-45.e3.
- [CrossRef] [PubMed] [Google Scholar]
- CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(Suppl 1):IV1-96.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with pre-and post-operative seizures in 1033 patients undergoing supratentorial meningioma resection. Neurosurgery. 2017;81:297-306.
- [CrossRef] [PubMed] [Google Scholar]
- Early and late postoperative seizures in meningioma patients and prediction by a recent scoring system. Cancers (Basel). 2021;13:450.
- [CrossRef] [PubMed] [Google Scholar]
- A Practical overview on the molecular biology of meningioma. Curr Neurol Neurosci Rep. 2020;20:62.
- [CrossRef] [PubMed] [Google Scholar]
- The surgical risk factors of giant intracranial meningiomas: A multi-centric retrospective analysis of large case serie. Brain Sci. 2022;12:817.
- [CrossRef] [PubMed] [Google Scholar]
- Brain tumors and epilepsy: Pathophysiology of peritumoral changes. Neurosurg Rev. 2009;32:274-84.
- [CrossRef] [PubMed] [Google Scholar]
- Seizures in supratentorial meningioma: A systematic review and meta-analysis. J Neurosurg. 2016;124:1552-61.
- [CrossRef] [PubMed] [Google Scholar]
- Letter: Neurosurgery and coronavirus (COVID-19) epidemic: Doing our part In: Neurosurgery. Vol 87. 2020. p. :E48-9. Erratum in: Neurosurgery 2020;87:426
- [CrossRef] [PubMed] [Google Scholar]
- Use of antiepileptic drugs as prophylaxis against posttraumatic seizures in the pediatric population: A systematic review and meta-analysis. Neurosurg Rev. 2023;46:49.
- [CrossRef] [PubMed] [Google Scholar]
- Multicentric and diffuse recurrences of meningiomas. Br J Neurosurg. 2020;34:439-46.
- [CrossRef] [PubMed] [Google Scholar]







