Translate this page into:
Navigating a grey zone: Neuromonitoring in the management of temporary internal carotid artery occlusion techniques - A case series and literature review
*Corresponding author: Rajeeb Kumar Mishra, Department of Neuroanesthesia and Neurocritical Care, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India. litu86@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma P, Malla S, Mishra RK, Surve R, Pendharkar HS, Kalgudi P. Navigating a grey zone: Neuromonitoring in the management of temporary internal carotid artery occlusion techniques - A case series and literature review. J Neurosci Rural Pract. 2024;15:484-490. doi: 10.25259/JNRP_36_2024
Abstract
There is a dearth of clearly defined thresholds to guide the application of neuromonitoring modalities in temporary vessel occlusion techniques. We report a case series exploring the utility of various neuromonitoring techniques during temporary vessel occlusion procedures. We conducted a retrospective chart review of patients, who underwent temporary vessel occlusion procedures over a two-year period and examined the neuromonitors employed in temporary vessel occlusion procedures including balloon occlusion test (BOT). We found complete details of nine patients, who were managed with the use of the following neuromonitors: cerebral oximetry, electrical activity monitors, evoked potential monitors, and transcranial Doppler. A literature search identified other studies reporting utilization of adjuvant neuromonitoring in these procedures. Although multiple sensors could be concurrently attached to patients without impeding image acquisition in patients undergoing BOT, our review of the literature and our own findings revealed a lack of consistent correlation with outcome, particularly concerning near-infrared spectroscopy values.
Keywords
Neuroradiology
Near-infrared spectroscopy
Balloon test occlusion
Cerebral collateral circulation
Neuromonitoring
INTRODUCTION
The evaluation of tolerance to permanent vessel occlusion typically involves a temporary balloon occlusion test (BOT) within the angiography suite. Manual compression or occlusion techniques in the operation theater (OT) are alternatives. This ensures collateral blood flow adequacy in the circle of Willis arteries, which prevents the risk of acute ischemic events. The need for such testing arises in various clinical scenarios, including wide-neck giant aneurysms, trauma, and cranial or cervical tumors involving the internal carotid artery (ICA).[1] However, the conventional test based only on clinical testing carries a stroke risk of 5–40%[2] and is influenced by anatomical variations and hemodynamic factors. Thus, it is often supplemented with radiographic techniques such as venous phase delay assessment, which are based on the assumption that patients who exhibit symmetry within the venous phase during a BOT have adequate intracranial collateral circulation and will withstand the loss of the parent vessel.[3] This can be further supplemented with monitoring of cerebral oxygenation, electrophysiological monitors, and bedside cerebral blood flow (CBF) monitoring. As a result, the anesthesiologist’s role in the BOT procedure specifically and other temporary occlusion test procedures more broadly has become increasingly important.
We present a retrospective case series that describes the outcomes of temporary occlusion test procedures and the viability of incorporating different bedside adjuncts to supplement angiographic assessment and/or clinical testing, while also highlighting the lack of standardized cutoffs in literature for interpreting the adequacy of collateral circulation with these monitors.
MATERIALS AND METHODS
A retrospective analysis of the medical records of patients, who underwent temporary vessel occlusion procedures and received additional neurological monitoring between February 2021 and February 2023 was conducted. We found data from nine patients whose findings were fully documented and who received some form of bedside monitoring such as transcranial Doppler (TCD) assessment, regional cerebral oxygen saturation (rSO2) monitoring with near-infrared spectroscopy (NIRS) or NIRS in combination with processed electroencephalography (EEG). Table 1 displays details of the nine patients with respect to the indication of BOT, adjuncts employed during monitoring, the results of angiographic and clinical testing, and the outcome for each patient. Cases 1 and 2 [Table 1] were performed in the OT using temporary ligation techniques. An exemption from the Institute Ethics Committee was obtained based on the retrospective nature of the data collection.
| Case No. | Diagnosis and Indication for occlusion testing | Result of clinical testing | Venous phase delay (seconds) | Outcome of surgery/interventional procedure post BOT | Adjunct Neuromonitoring used during the vessel occlusion procedure | |||
|---|---|---|---|---|---|---|---|---|
| Type of neuromonitoring used | Findings of the first adjunct monitor | Findings of the second adjunct monitor | ||||||
| 1 | Left cavernous ICA giant aneurysm planned for ICA ligation | Passed BOT | Normotension-1 Hypotension-1.5 |
Ligation of left cervical ICA-Postoperative CT angiogram showed no filling of the aneurysm and perfusion CT showed no reduction in perfusion compared with the right hemisphere | Evoked potential monitoring (MEP and SSEP) | Preserved MEPs [Figure 1] | Disappearance of SSEP with hypotension → returned to baseline with normalisation of blood pressure [Figure 1] | |
| 2 | Right superior hypophyseal artery aneurysm planned for ICA ligation | Passed temporary ligation of CCA | Not applicable. | Post operatively patient developed left upper limb and lower limb weakness and was discharged with 3/5 motor power on MRC scale. CT findings in [Figure 2]. | Bilateral cerebral oxygenation (rSO2) and cerebral blood flow velocity (TCD) | 1. Drop in ipsilateral values of rSO2 by 9% during normotension | MCA mean velocity -Ipsilateral side reduced | |
| 2. Maximum interhemispheric difference was 8%. | Contralateral side reduced | |||||||
| 3 | Right Middle cranial fossa base lesion (Histopathology-Hemangioma) with ipsilateral ICA involved by lesion | Passed BOT | Normotension-0.5 Hypotension-Data not available |
No fresh post-op deficits after lesion resection | Bilateral cerebral oxygenation (rSO2) | No significant change (3% drop from baseline), interhemispheric difference between 3 and 4% | - | |
| 4 | Left cavernous sinus meningioma with ipsilateral ICA involved by lesion | Passed BOT | Normotension-0.5 | Vessel not sacrificed. Post-op new onset vision deficit-Left eye hand movement close to face |
Bilateral cerebral oxygenation (rSO2) and cerebral electrical activity by (PSI) | 1. Normotension- Maximum drop in ipsilateral rSO2 8% | PSI values unchanged between 92 and 94%. | |
| Hypotension-0.5 | 2. Hypotension- No significant change | |||||||
| 3. Maximum interhemispheric difference rSO2 8% | ||||||||
| 5 | Left supraclinoid ICA giant aneurysm planned for ICA ligation | Passed BOT | Normotension-0.5 | Underwent ICA ligation with no fresh post-op deficits | Bilateral NIRS and PSI | 1. Normotension-Maximum drop in rSO2 on ipsilateral side 3% | PSI between 88 and 94% throughout. | |
| Hypotension-1 | 2. No change with hypotension | |||||||
| 3. No significant interhemispheric difference. | ||||||||
| 6 | Left cavernous ICA giant aneurysm planned for either parent vessel occlusion/Flow diverter/ICA ligation | Passed BOT | Normotension-0 Hypotension-1 |
Flow diverter placement abandoned due to technical difficulty; no deficits post-operatively | Bilateral NIRS and PSI | 1. Normotension-No significant change | PSI between 91 and 95%. | |
| 2. Hypotension-Maximum drop in ipsilateral rSO2 8% | ||||||||
| 3. Maximum interhemispheric difference in rSO2 values 6% during hypotensive challenge. | ||||||||
| 7 | Right clinoidal meningioma with ipsilateral ICA encased by lesion | Passed BOT | Normotension-0.33 Hypotension-1 |
Vessel persevered intraoperatively, no deficits post-op | Bilateral NIRS | 1. Normotension-aximum drop in ipsilateral rSO2 4% | ||
| 2. Hypotension-15% | ||||||||
| 3. Interhemispheric difference maintained | ||||||||
| 8 | Left supraclinoid ICA saccular aneurysm planned for ICA ligation | Passed BOT | Normotension-0 Hypotension-1 |
Post ICA ligation-No deficits | Bilateral NIRS and PSI | 1. Normotension-maximum drop in ipsilateral rSO2 8–10% | PSI values unchanged between 90% and 94%. | |
| 2. Hypotension-Drop of 12% after | ||||||||
| 3. No change in interhemispheric difference | ||||||||
| 9 | Left ICA cavernous segment aneurysm planned for either ICA ligation or parent vessel occlusion | Passed BOT | Normotension-1 Hypotension-1 |
Currently not operated | Bilateral NIRS and PSI | 1. Normotension-Maximum drop in ipsilateral rSO2 7% | PSI values unchanged between 88% and 94%. | |
| 2. Hypotension-8% drop | ||||||||
| 3. Interhemispheric | ||||||||
| difference up to 5% maintained throughout. | ||||||||
ICA: Internal carotid artery, MCA: Middle cerebral artery, BOT: Balloon occlusion test, TCD: Transcranial Doppler, SSEP: Somatosensory evoked potential, MEP: Motor evoked potential, FND: Focal neurological deficit, CT: Computed tomography, NIRS: Near infrared spectroscopy, CCA: Common carotid artery, MRC: Medical research council, rSO2: Regional cerebral oxygen saturation, PSI: Patient state index
DISCUSSION
In the first case, temporary clamping along with somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP) monitoring was performed, which remained intact under normotensive conditions. When hypotension was induced, the SSEP signal disappeared [Figure 1], but the MEP signal remained intact. Once blood pressure (BP) normalized, the SSEP signal reappeared. Overall, the patient tolerated the vessel ligation procedure well, and no focal deficits were observed. This case highlights the utility of confirming the integrity of sensory and motor pathways during temporary vessel occlusion testing. There is evidence supporting MEP monitoring, as it exhibits greater sensitivity compared to SSEP in detecting cerebral ischemia.[4] On the other hand, despite the absence of significant SSEP changes, patients may still experience motor impairments after surgery. Takamura et al. studied 32 patients, with 12.5% BOT-positive results, undergoing surgery with MEP monitoring.[5] The cutoff value for MEP amplitude indicating BOT positivity was determined to be a reduction of >80% from the baseline (100% sensitivity and 100% specificity), which was significantly higher compared to EEG monitoring (100% sensitivity and 72.0% specificity).[5] Another study investigating temporary occlusion during aneurysm surgery found that out of 97 patients, in 42 cases (30 with middle cerebral artery [MCA] aneurysms and 12 with ICA aneurysms) SSEP disappeared during the occlusion period. Thirty-nine eventually recovered their baseline SSEP levels after recirculation, and no postoperative complications were observed in them. However, for the remaining three patients, SSEP did not recover even after recirculation, and all three patients experienced post-operative complications.[6] In our patient, MEP did not disappear, and SSEP returned to baseline after correction of hypotension, thus the decision to go ahead with parent vessel ligation was taken. It is important to remember that the induction of hypotension during BOT can have an impact on cerebral arteriolar tone, either through pharmacological effects or by triggering autoregulatory mechanisms leading to false negative rates of up to 5–15%, highlighting the need for additional confirmatory testing.
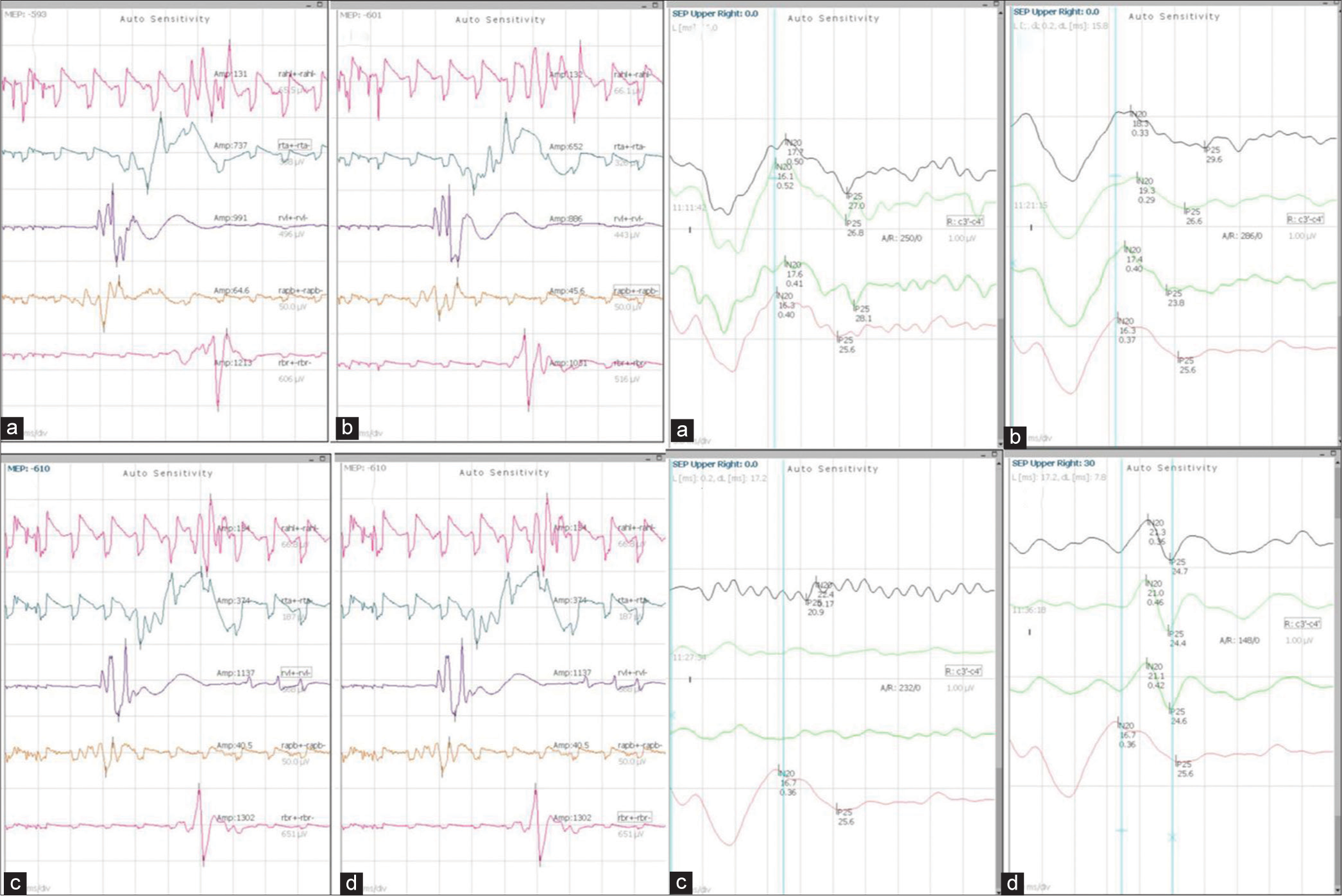
- Findings of case 1 with MEP and SSEP monitoring: Left panel: MEP findings (a) Pre clamp (b) Post clamp (c) After induction of hypotension (d) After normotension. Right Panel: SSEP findings (a) Pre clamp (b) Post clamp (c) After induction of hypotension (d) After normotension. MEP: Motor evoked potentials, SSEP: Somatosensory evoked potential.
In case 2, after placing the temporary clamp on the common carotid artery, NIRS measurements dropped from 84 to 75 on the side of occlusion. Simultaneously, TCD mean flow velocities decreased from 54 to 48 (12% drop) in the M1 MCA but eventually returned to baseline. The maximum interhemispheric rSO2 difference reached 8% after 10 min of occlusion. Over 10 min, this hemispheric difference reduced probably signifying compensation from collaterals. Clinically, the patient appeared stable, thus the surgeon proceeded with vessel sacrifice. However, post-procedure, the patient developed contralateral weakness, with a power of 3/5 in both upper and lower limbs at discharge. A computed tomography scan revealed patchy watershed infarcts in the MCA territory on the affected side [Figure 2], likely due to embolic stroke resulting from clamp placement. Thus, techniques focusing on the assessment of global cerebral function rather than a single territory of the ICA may be more useful for the identification of such complications. Sorteberg et al. examined 136 patients using TCD-guided BOT.[7] They found that a significant drop in MCA velocity during balloon occlusion can serve as an indicator of the patient’s tolerance to ICA sacrifice. Patients with a drop to 65% or more of baseline values are likely to tolerate the procedure, while those with a drop to 54% or less are at higher risk of developing infarction.[7] However, assessment with TCD-based monitoring may fail to identify ischemia in the watershed zones.
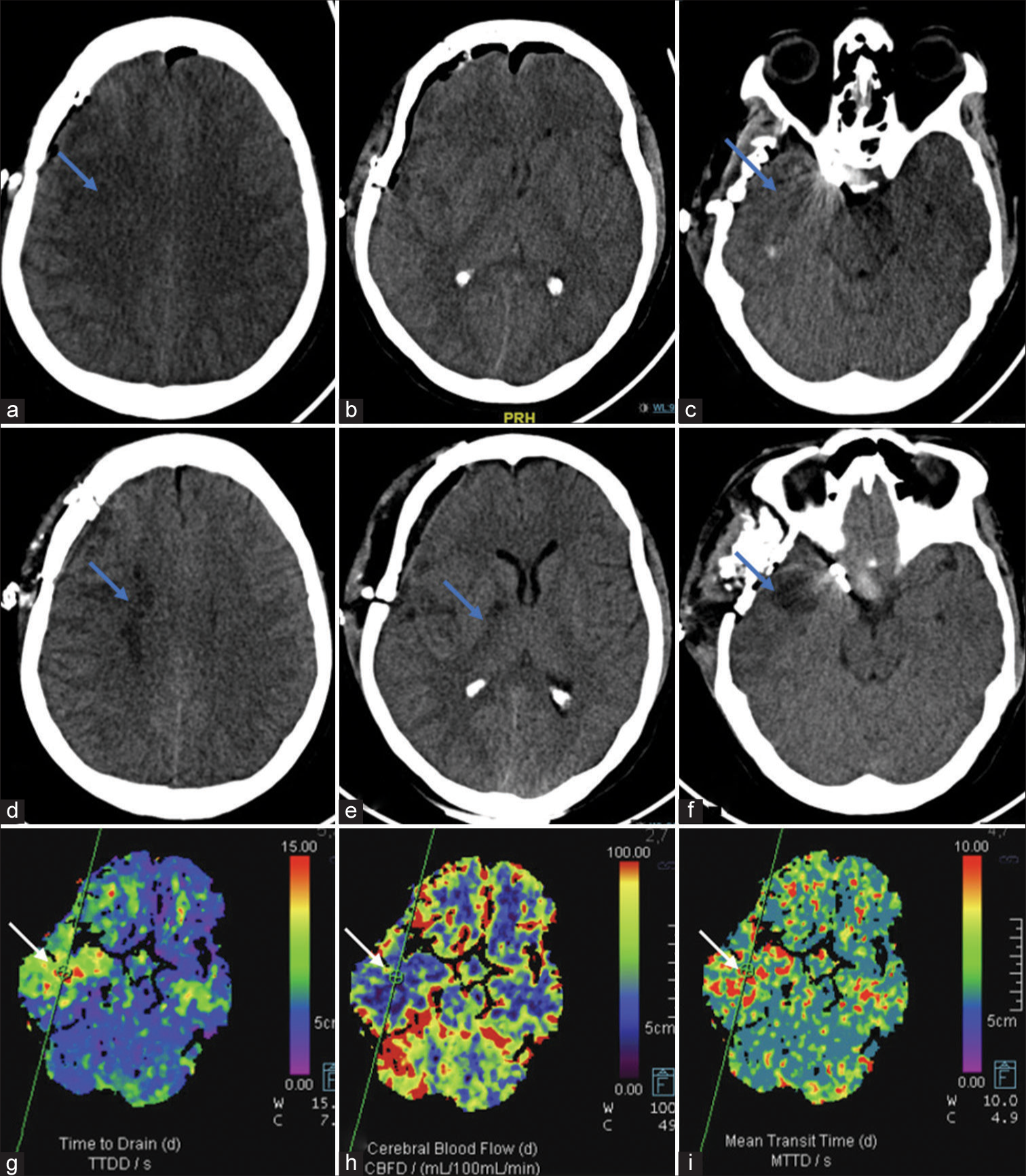
- Findings of Case 2: (a-c) Day 1 post-clipping non-contrast computed tomography (NCCT) shows right pterional craniotomy with pneumocephalus. Subtle hypodensities (blue arrows) are seen in the right frontal lobe and anterior temporal lobe. (d-f) Day 4 patient developed left upper limb and lower limb weakness and repeat NCCT shows well-developed infarcts (blue arrows) in the right internal watershed zone, posterior limb internal capsule and anterior temporal lobe. (g-i) Concomitant computed tomography perfusion was performed, which revealed markedly decreased cerebral blood flow, increased time to drain, and mean transit time indicative of infarcted tissue (blue arrows).
Limited studies have investigated cerebral oxygenation monitors in BOT. A study in nasopharyngeal carcinoma patients found that all 11 BOT-positive patients demonstrated a significant drop in rSO2 on the tested side. Through receiver operating characteristic curve analysis, they determined that a change in rSO2 (∆rSO2) of 5% was the most suitable cut-off value for detecting the BOT-positive group.[8] However, NIRS rSO2 monitoring was unable to detect false-negative results. One limitation we have observed is that pain may induce elevations in BP and subsequently increase in rSO2 values, which can confound clinical testing. In another study, simultaneous monitoring of rSO2 and technetium 99m-hexamethylpropyleneamine oxime single-photon emission computed tomography study was assessed during BOT, which revealed a significant correlation between ∆rSO2 and stump pressure.[9]
The hypothesis behind using oxygenation monitors is that in patients with inadequate collateral circulation, rSO2 is expected to be lower on the ipsilateral side after balloon occlusion, and the presence of hypoxia in patients who fail BOT should be reflected in the interhemispheric difference between rSO2 values. A concern regarding the use of these monitors is their potential to disrupt image acquisition. However, based on our experience, as demonstrated in Figure 3, the use of NIRS does not interfere with image acquisition.
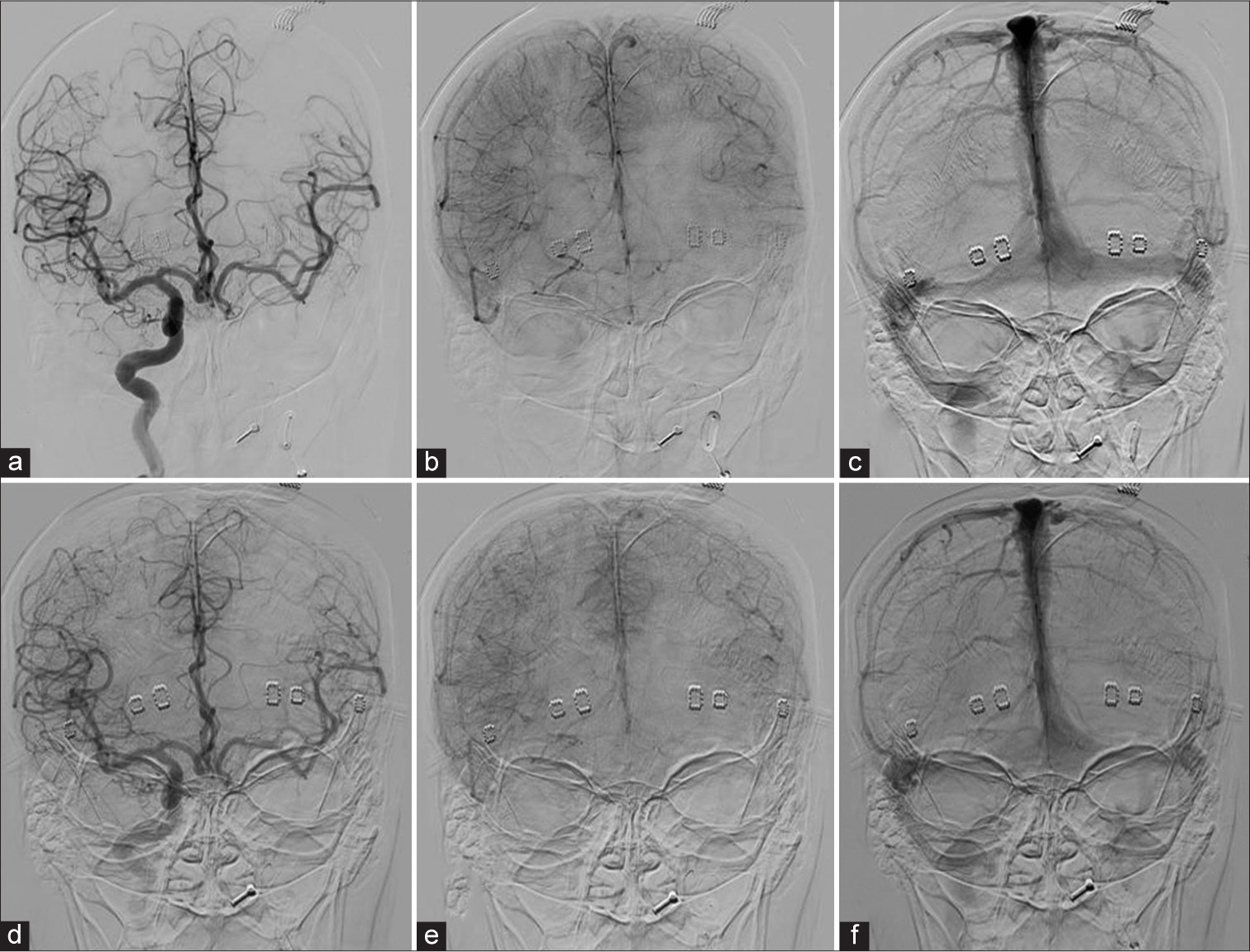
- Venous phase assessment of case 9: (a) Angiogram AP view, injected by right ICA shows arterial phase. (b and c) Early and late venous phases, respectively, demonstrate perfect synchronous venous filling of the left hemisphere through Acom. (d) The arterial phase of the injection through right ICA during hypotensive challenge demonstrates excellent cross-filling through Acom. (e and f), Early and late venous phases, respectively, show symmetry of venous filling of left ACA and MCA territories. ICA: Internal carotid artery, Acom: Anterior communicating artery, ACA: Anterior cerebral artery, MCA: Middle cerebral artery.
In our series, eight patients (cases 2–9) undergoing temporary vessel occlusion exhibited varied degrees of reduction (3–10%) in ipsilateral rSO2 values under normotensive conditions. In cases 7 and 8, a drop of up to 12–15% was observed post hypotension. However, none of these changes in rSO2 values were associated with a false positive or a false-negative BOT result. Therefore, it is crucial to establish a cut-off value for ∆rSO2 in patients undergoing BOT and correlate these values with angiographic evidence of positive or negative BOT.
The EEG changes have been shown to correspond to CBF changes in carotid endarterectomy (CEA). During CEA, a reduction in bispectral index (BIS) has been observed when cerebral ischemia occurs due to ICA cross-clamping. Thus, the use of bilateral BIS can aid in detecting a difference between hemispheres with BOT. Harclerode et al. reported a reduction in ipsilateral BIS with each balloon inflation during balloon-assisted cerebral aneurysm coiling in a case report.[10] To date, no large studies have evaluated the utility of processed EEG monitors during BOT.
The complications that can occur during temporary vessel occlusion testing also need to be considered. Excessive manipulation during the procedure can lead to intraoperative thrombus generation or vessel dissection. Thus, it is important to prevent hypotension to maintain optimal systemic circulation during occlusion. However, hypotensive challenge is an integral aspect of clinical testing during BOT. Neuromonitoring can serve a dual role: Assessing collateral integrity and identifying complications early to facilitate prompt treatment.
While our case series provides insights into the utility of neuromonitoring during temporary vessel occlusion testing, limitations should be considered. The retrospective nature of the data collection introduces inherent biases and limitations, such as the completeness and correctness of data. Each modality has its own limitations and strengths, and a multimodal approach may yield more comprehensive information.
CONCLUSION
Our series has emphasized the varied findings in BOT studies, merging them with our experiences. As technology advances and clinical practices evolve, integrating neuromonitoring into routine procedures can enhance patient safety, but without well-defined cutoff values for interpretation, the use of individual neuromonitoring devices in BOT can potentially generate confusion among clinicians. This highlights the need for continued research and standardization of neuromonitoring protocols to enhance the accuracy of these techniques during temporary vessel occlusion testing.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Stroke risk after abrupt internal carotid artery sacrifice: Accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829-43.
- [Google Scholar]
- Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005;26:2602-9.
- [Google Scholar]
- Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg. 2004;100:389-99.
- [CrossRef] [PubMed] [Google Scholar]
- Motor evoked potential monitoring can evaluate ischemic tolerance to carotid artery occlusion during surgery. J Clin Monit Comput. 2021;35:1055-62.
- [CrossRef] [PubMed] [Google Scholar]
- Permissible temporary occlusion time in aneurysm surgery as evaluated by evoked potential monitoring. Neurosurgery. 1993;33:434-40.
- [CrossRef] [PubMed] [Google Scholar]
- Angiographic balloon test occlusion and therapeutic sacrifice of major arteries to the brain. Neurosurgery. 2008;63:651-61.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon test occlusion of internal carotid artery in recurrent nasopharyngeal carcinoma before endoscopic nasopharyngectomy: A single center experience. Front Oncol. 2021;11:674889.
- [CrossRef] [PubMed] [Google Scholar]
- An additional monitoring of regional cerebral oxygen saturation to HMPAO SPECT study during balloon test occlusion. Stroke. 1999;30:407-13.
- [CrossRef] [PubMed] [Google Scholar]
- Bispectral index detects intraoperative cerebral ischaemia during balloon assisted cerebral aneurysm coiling. F1000Res. 2014;2:225.
- [CrossRef] [Google Scholar]







