Translate this page into:
Recurrent demyelination attacks after BNT162b2 vaccination: Two case reports and literature review
*Corresponding author: Asuman Orhan Varoglu, Department of Neurology, Istanbul Medeniyet University, Istanbul, Turkey. asumanorhan69@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Orhan Varoglu A, Hosver B, Güllüoğlu Torun Z. Recurrent demyelination attacks after BNT162b2 vaccination: Two case reports and literature review. J Neurosci Rural Pract. 2024;15:498-502. doi: 10.25259/JNRP_496_2023
Abstract
We describe two cases with recurrent demyelinating attacks following BioNTech BNT162b2 immunization. No reports of any recurring demyelinating attack cases connected to COVID-19 immunization. Case-1: A 37-year-old male patient was admitted due to diplopia and ptosis. Neurological examination showed isolated oculomotor cranial paralysis on the right side. The second dose of BNT162b2 was given 10 days ago. On T2- and fluid-attenuated inversion recovery (FLAIR)-weighted magnetic resonance imaging (MRI), hyperintense lesions were seen in the pons on T1, with no evidence of contrast enhancement. We diagnosed the patient with central pontine myelinolysis associated with BNT162b2. Six months later after the first attack, a new lesion appeared in the same region. Case-2: He applied to the hospital at the age of 57 years, complaining of numbness in his hands and feet, confusion, and cooperation disorder. During the neurological assessment, apathy, cooperation, and orienting disorders were found. Hyperintense lesions were seen in both hemispheres, with cortico-subcortical localization on MRI. The patient received the BNT162b2 two weeks ago. After the initial attack six months ago, new clinical signs and an increase in demyelinating lesions were found. Despite early corticosteroid treatment, BNT162b2 immunization may be associated with repeated demyelination attacks. In patients with diffusion restriction on MRI, we might suggest using corticosteroid therapy for approximately one year, a lot longer than the literatures suggested.
Keywords
Demyelination lesion
Central pontine myelinolysis
Osmotic demyelination
BNT162b2
COVID-19 vaccination
INTRODUCTION
The most prevalent cause of osmotic demyelination syndrome (ODS) is fast hyponatremia correction.[1] The COVID-19 infections can cause demyelination lesions in the central nervous system.[2,3] Following the BNT162b2 administration, certain adverse responses have also been reported.[3] To the best of our knowledge, only two patients with ODS caused by COVID-19 infection have been reported. One is a case of extrapontine myelinolysis, whereas the other is a case of central pontine myelinolysis (CPM).[4,5]
No case of recurrent CPM and demyelination lesions associated with COVID-19 vaccination has been reported. We report two cases of recurrent demyelinating attacks after vaccination with BioNTech BNT162b2.
CASE REPORT
Case-1
For the past four days, a 37-year-old male patient in our hospital has been complaining of diplopia and ptosis in the right eye. Isolated oculomotor cranial paralysis on the right side was discovered on neurological evaluation. The second dose of the BNT162b2 vaccine was given 10 days ago. All biochemical data were normal. The viral panel, culture, and biochemistry results of the cerebrospinal fluid (CSF) investigations were normal. On diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI), a very small pons region showed restriction of diffusion. On the T2- and fluid-attenuated inversion recovery (FLAIR)-weighted MRI, a hyperintense lesion was seen in the central pontine area [Figure 1a]. Cerebral and cervical angiography was normal. Anti-myelin oligodendrocyte glycoprotein (anti-MOG) and the oligoclonal band were both negative in the CSF. Anti-aquaporin-4 (neuromyelitis optica spectrum disorder) and all collagen tissue disease markers were negative. Uveitis and oral aphthous ulcerations were not found. Human leukocyte antigen B51 (HLADR B1) and pathergy test were also negative. Hence, we ruled out Behcet’s disease. An MRI spectroscopic imaging and positron emission tomography-computed tomography imaging of the whole body and brain were not compatible with brain malignancies. The patient was diagnosed with CPM. Methylprednisolone (IVP, 1000 mg/day) therapy was started for 10 days. At this stage, the hyperintense lesions in the pontine area on T2-weighted and FLAIR-weighted MRI dramatically shrunk from their initial size and practically disappeared [Figure 1a]. After three months of clinical complaints, the patient recovered completely, and corticosteroid treatment was stopped.
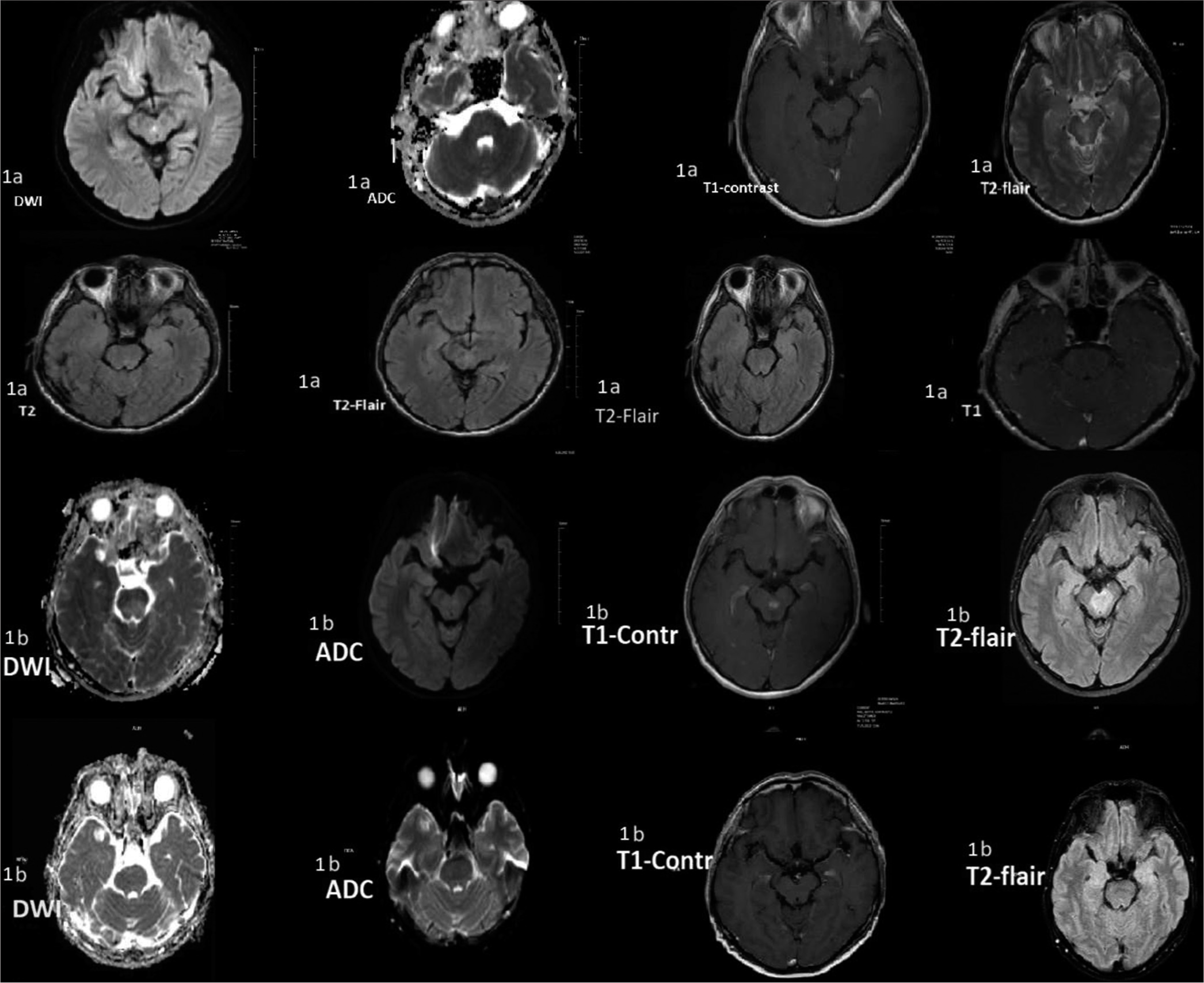
- Case-1 (a) Restriction of diffusion was seen on diffusion-weighted imaging (DWI), magnetic resonance imaging (MRI) in a very small pons area. Apparent diffusion coefficient (ADC)-weighted MRI showed a hypointense lesion in a very small pons area. T1-weighted MRI with contrast showed an isointense lesion. On T2-weighted and fluid-attenuated inversion recovery (FLAIR)-weighted MRI, hyperintense lesions were seen in the central pontine area indicating central-pontine osmotic demyelination. After the treatment of approximately 15 days, On T2-weighted and FLAIR-weighted MRI, the hyperintense lesions in the central pontine region reduced considerably from their initial size and almost disappeared at this point. Case-1 (b) After the first admission six months later, restriction of diffusion was also seen on DWI MRI in the pons area. The ADC-weighted MRI showed a hypointense lesion in the same area very slightly. T1-weighted MRI with contrast (T1-Contr) showed enhancement of contrast in the same lesion. On T2-weighted and FLAIR-weighted MRI, hyperintense lesions were seen in the central pontine area. After the initial MRI of approximately three months, the pons lesion was shown to have no diffusion restriction in DWI MRI, and ADC MRI was normal. In the same region, T1-weighted MRI with contrast revealed no enhancement of contrast. On T2-weighted MRI, the hyperintense lesions in the central pontine region were not seen.
Three months after the corticosteroid therapy was stopped, the patient was brought to our hospital with the same complaints. An MRI discovered a contrast-enhancing CPM lesion in the same location [Figure 1b]. After 1000 mg/day of methylprednisolone treatment, lesions shrunk significantly on MRI, and his clinical signs improved dramatically.
Case-2
At the age of 57 years, he applied to the emergency service with complaints of numbness in the hands and feet, disorientation, and cooperation disorder. Cooperation and orientation disorder and apathy were discovered during the neurological evaluation. In contrast-enhanced MRI, hyperintense lesions were seen in both hemispheres [Figure 2a]. We discovered the existence of the BNT162b2 vaccine in the medical past around two weeks ago. Protein 0.9 mg/dL (reference range 0.15–0.45 mg/dL) and Cell 15/mm3 (reference range 0–5/mm3) were found in the CSF investigation. Paraneoplastic, limbic encephalitis, and herpes simplex polymerase chain reaction tests were also negative. Anti-aquaporin-4, anti-MOG, and the oligoclonal band were negative. Diseases of the collagen tissue were eliminated. The patient was diagnosed with vaccine-induced autoimmune demyelination lesion disease. Methylprednisolone (IVP, 1000 mg/day) therapy for 10 days and oral prednisolone therapy as maintenance for 3–4 months at follow-up were given. The patient’s clinical recovery completely improved.
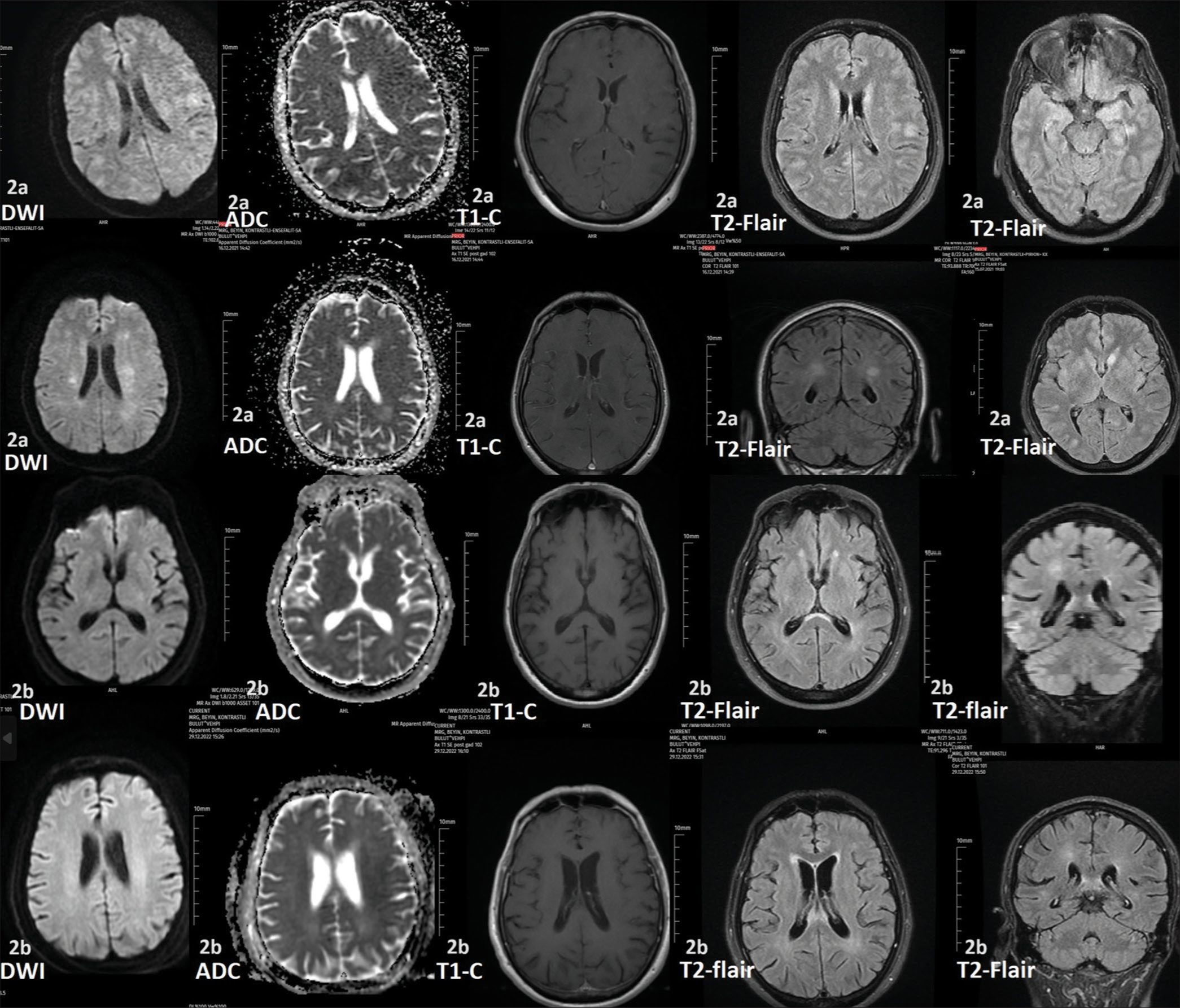
- Case-2 (a) Restrictions of diffusion were seen on diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI) in the bilateral frontal lobes, bilateral frontal lobes’ cortico-subcortical localization, bilateral caudate nucleus head area, and apparent diffusion coefficient (ADC)-weighted MRI is within normal limits. On T1-weighted MRI, not all lesions showed enhancement. On T2-weighted and fluid-attenuated inversion recovery (FLAIR)-weighted MRI revealed the existence of hyperintense lesions in the bilateral frontal lobes, bilateral frontal lobes’ cortico-subcortical localization, bilateral caudate nucleus head, bilateral lentiform nuclei, bilateral insular cortex, and bilateral parahippocampal gyrus localization. At the bottom of the image following therapy for around 15 days, the hyperintense lesions in the aforementioned places on T2-weighted and FLAIR-weighted MRI showed a modest reduction in size from their initial size. Case-2 (b) DWI, ADC-weighted MRI, and T1-weighted MRI were normal limits. Hyperintense lesions were seen on T2-weighted and FLAIR-weighted MRI in the corpus splenium area, bilateral cortico-subcortical localization, and frontal lobes. At the bottom of the picture after treatment for about 15 days, the hyperintense lesions in the aforementioned locations on T2-weighted and FLAIR-weighted MRI exhibited a small reduction in size from their initial size.
The patient applied to our outpatient clinic again about 6–7 months following the initial attack, complaining of instability and worsening walking. A neurological evaluation indicated paraparesis (4/5) and deficient cerebellar testing. Lesions did not enhance contrast in MRI scans [Figure 2b]; however, there was an increase in the number of lesions. During the CSF analysis, 0.55 mg/dL of protein and 7/mm3 of cells were found. Methylprednisolone (IVP) was re-administered, and the clinical of the patient also greatly improved.
DISCUSSION
The ODS has been described as occurring in normal Na levels, hypernatremia, hypokalemia, and hyperglycemia. When contemplating the development of CPM, the presence of hyponatremia should first be evaluated.[1] In our patient, serum Na levels were normal, and he did not have any disease but received the second dose of the BioNTech BNT162b2 vaccine approximately 10 days ago. The hyperintense lesions decreased dramatically and nearly disappeared after two weeks. It has been noted that radiological alterations normally improve with clinical improvement and that they can occasionally become permanent as a surprise.[1] In our case, as reported in ODS cases in the literature,[1] hyperintense lesions were observed to disappear with clinical improvement. In our patient, isolated oculomotor cranial paralysis on the right side was identified. We thought that different clinical symptoms in ODS were less relevant for prognosis than early diagnosis and treatment.
Demyelination lesions in the central nervous system induced by COVID-19 mRNA-based vaccination have been documented. With corticosteroid therapy, demyelination lesions were considerably regressed in all of these patients, and neurological evaluations were also very good.[2] No demyelination lesions were seen among the published patients in the central pontine region. Furthermore, repeated demyelinating lesions and clinical attacks have not been reported so far. Unlike previously published reports, we present two cases with repeated demyelination attacks due to BNT162b2 immunization despite corticosteroid treatment. Some authors have claimed that immunization against COVID-19 in multiple sclerosis patients is not linked to relapse or a bad prognosis.[6] However, Havla et al.[7] reported that the Pfizer BNT162b2 vaccine induced the patient’s first manifestation of multiple sclerosis attacks. To the best of our knowledge, our cases are the first instances in which repeated demyelinating attacks due to the BioNTech BNT162b2 vaccine. The DWI-weighted MRI revealed diffusion restrictions even though both patients had normal apparent diffusion coefficient -weighted MRIs at the time of their initial clinical presentation. We thought that therapy should be continued for a longer amount of time, such as 9–12 months, among patients who had diffusion restrictions on DWI-weighted MRI at the time of their initial attack since they may have a higher risk of relapsing.
CONCLUSION
It may be helpful to keep in mind that BioNTech BNT162b2 immunization may result in repeated demyelinating attacks. We suggest that it is necessary to continue corticosteroid therapy for a much longer period, as demyelinating attacks may develop repeatedly despite early and effective therapy in these cases.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Osmotic demyelination syndrome: Clinical, neuroimaging characteristics, and outcomes in a series of 18 cases. Biomed Res Int. 2021;28:9944632.
- [CrossRef] [PubMed] [Google Scholar]
- Miller Fischer and posterior reversible encephalopathy syndromes post COVID-19 infection. Neurosciences (Riyadh). 2021;26:295-9.
- [CrossRef] [PubMed] [Google Scholar]
- Aseptic pleocytosis eight days after the first dose of a vector-based SARS-CoV-2 vaccine. J Neurosci Rural Pract. 2023;14:154-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hyponatremia and extrapontine myelinolysis in a patient with COVID-19: A case report. Clin Case Rep. 2021;9:e04463.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral imaging in patients with COVID-19 and neurological symptoms: First experience from two university hospitals in northern Germany. Rofo. 2021;193:667-71.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler. 2021;27:864-70.
- [CrossRef] [PubMed] [Google Scholar]
- First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022;269:55-8.
- [CrossRef] [PubMed] [Google Scholar]







