Translate this page into:
Combined transchoroidal and subchoroidal approach for resection of a large hemorrhagic epithelial cyst: Expanding the operative corridor to the third ventricle
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Surgical resection of the third ventricular lesions can be challenging, due to the depth of the operative corridor. Small or medium-sized lesions can be accessed through an interhemispheric transcallosal transchoroidal approach to the third ventricle.[1] The transchoroidal approach has become the preferred route for third ventricular lesions, since widening the foramen of Monro posteriorly by opening the naturally occurring choroidal fissure minimizes the risk of neurovascular injury.[2] However, the transchoroidal approach may be insufficient to access large third ventricular lesions, particularly those which do not readily collapse with internal debulking. We describe a rare case of a large, hemorrhagic epithelial cyst of the third ventricle which was resected through a transcortical, combined transchoroidal, and subchoroidal approach.
A 53-year-old male presented with headache, nausea, vomiting, and seizure. Brain computed tomography showed obstructive hydrocephalus of both lateral ventricles secondary to a third ventricular mass. Bilateral external ventricular drains were placed emergently. Additional evaluation with brain magnetic resonance imaging (MRI) showed a 3.7 cm × 3.6 cm × 3.5 cm peripherally enhancing, hemorrhagic lesion arising from the septum pellucidum and superior aspect of the third ventricle [Figure 1a–c]. We then proceeded with microsurgical resection.
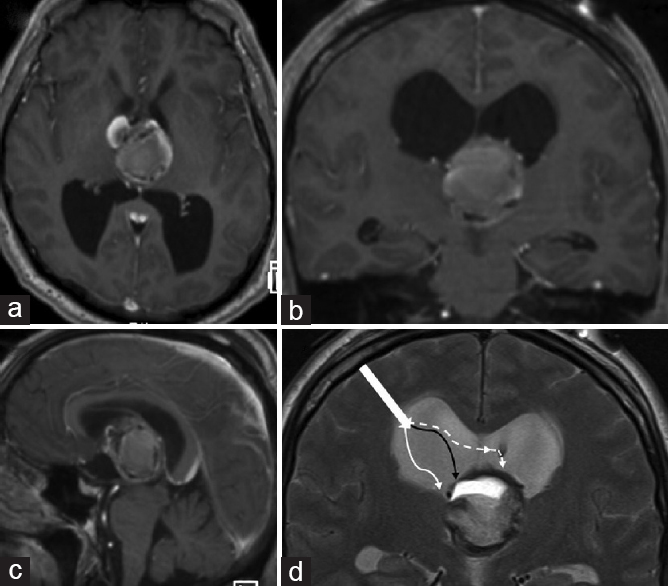
- Preoperative magnetic resonance imaging brain, T1-weighted sequence with gadolinium contrast, (a) axial, (b) coronal, and (c) sagittal views, shows a 3.7 (anteroposterior) × 3.6 (transverse) × 3.5 (craniocaudal) cm lobulated, hemorrhagic lesion with heterogeneous peripheral enhancement which arises from the septum pellucidum and superior aspect of the third ventricle, (d) T2-weighted sequence, coronal view, shows the surgical approach taken to resect the lesion; after a transcortical approach to the right lateral ventricle, (blocked white arrow) we performed an ipsilateral combined transchoroidal (solid black arrow) and subchoroidal (solid white arrow) approach to the third ventricle, and further increased our exposure of the lesion by performing a septostomy and contralateral transchoroidal approach (dashed white arrow)
After patient's head was affixed in a Mayfield skull clamp, a right frontoparietal craniotomy was performed which was two-thirds anterior and one-third posterior to the coronal suture. We performed the right frontal corticotomy around the ventricular catheter and followed its tract into the right lateral ventricle. A Budde Halo brain retractor system was attached to the Mayfield skull clamp, and two fixed retractor blades were placed to maintain the transcortical corridor into the ventricle. The anterior portion of the lesion protruding through the foramen of Monro was dissected off of the fornix medially and the caudate laterally.
The ipsilateral choroidal fissure was then opened widely, cutting both the taenia fornicis medial to the choroid plexus and the taenia thalami lateral to the choroid plexus. We then skeletonized the right internal cerebral vein (ICV) in the velum interpositum and divided the septal vein, which allowed lateral transposition of the ICV. This completed the combined transchoroidal and subchoroidal approach to the third ventricle [Figure 1d]. The lesion was then resected in a piecemeal fashion using the standard microsurgical technique. A septostomy was performed, and a contralateral transchoroidal approach was taken to further increase visualization and facilitate dissection of the lesion off of the left-sided neurovascular structures. A gross total resection was achieved, and the thalamostriate veins and ICVs were preserved bilaterally.
Postoperative MRI showed no evidence of residual enhancing tumor [Figure 2]. The patient had an uncomplicated postoperative course and was discharged to a rehabilitation facility 2 weeks after surgery. At 2 months follow-up, the patient was functionally independent with a nonfocal neurological exam. His only symptom was occasional difficulty with short-term memory (modified Rankin Scale of 1). Histopathological analysis of the lesion revealed an epithelial cyst with the areas of hemosiderin deposition, cholesterol clefts, inflammatory cells (predominantly lymphocytes), and occasional multinucleated giant cells within the cyst wall, without features of a craniopharyngioma or malignancy. Therefore, the lesion was favored to be a hemorrhagic colloid cyst, although a definitive diagnosis of the specimen could not be made.
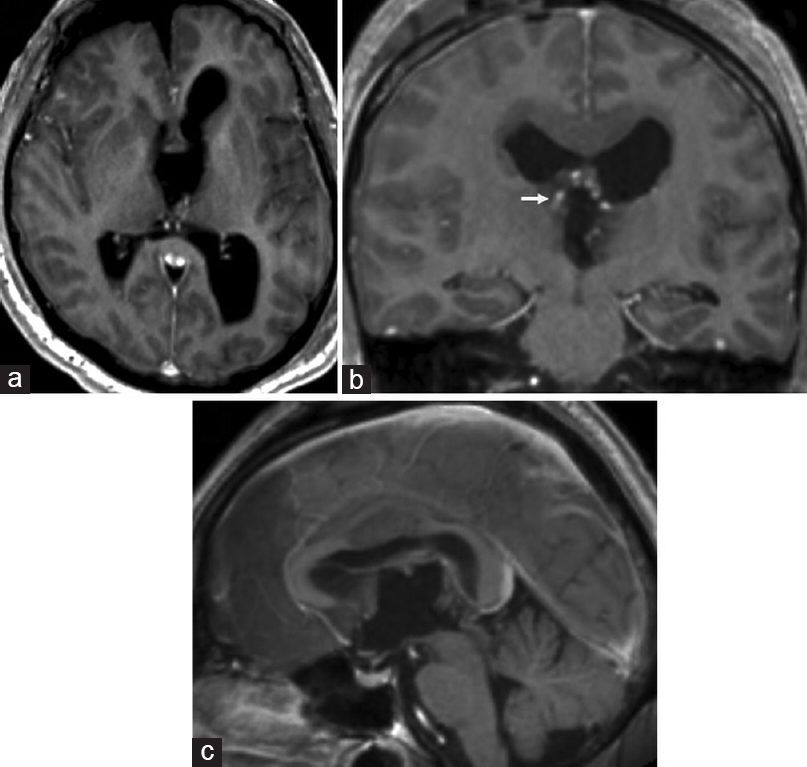
- Postoperative magnetic resonance imaging brain, T1-weighted sequence with gadolinium contrast, (a) axial, (b) coronal, and (c) sagittal views, shows no evidence of residual enhancing tumor, (b) the coronal view shows patency of the right thalamostriate vein (arrow)
In general, colloid cysts can be resected using endoscopic or microsurgical techniques.[3] However, hemorrhagic colloid cysts are exceedingly rare, and due to inflammation and fibrosis, may be more adherent to adjacent neurovascular structures.[4] The interhemispheric transchoroidal approach is generally favored for third ventricular lesions since the subchoroidal approach places the thalamostriate vein at risk.[5] For larger lesions, we favor a transcortical approach to the lateral ventricle over an interhemispheric one, since the former affords a wider operative corridor. Surgical access to the third ventricle can be substantially increased by combining the transchoroidal and subchoroidal approaches, ligating the septal vein, and mobilizing the ICV laterally. In addition, the thalamostriate vein can be preserved using this combined approach, thereby minimizing the risk of postoperative venous infarction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Interhemispheric transchoroidal approach to resect third ventricular teratoma. Neurosurg Focus 2016:40. 40 Video Suppl 1:2016.1.FocusVid. 15401
- [Google Scholar]
- Transchoroidal approach to the third ventricle: An anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42:1205-17.
- [Google Scholar]
- Combined endoscopic transforaminal-transchoroidal approach for the treatment of third ventricle colloid cysts. J Neurosurg. 2014;120:1471-6.
- [Google Scholar]
- Hemorrhagic colloid cyst: Case report and review of the literature. Asian J Neurosurg. 2013;8:162.
- [Google Scholar]
- Interhemispheric transcallosal subchoroidal fornix-sparing craniotomy for total resection of colloid cysts of the third ventricle. J Neurosurg. 2009;110:112-5.
- [Google Scholar]





