Translate this page into:
Isolated Sphenoid Sinus Lesions: Experience with a Few Rare Pathologies
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The sphenoid sinus is often neglected because of its difficult access. The deep position of the sphenoid sinus hinders early diagnosis of pathologies in that location. Delayed diagnosis can cause serious complications due to proximity to many important structures.
Objectives:
The aim of this study is to demonstrate different pathologies which can affect the sphenoid sinus and elucidate the findings.
Methods:
Cases of isolated sphenoid sinus lesions encountered in the neurosurgical setting which had rare pathologies are discussed. Pathologies such as Langerhans cell histiocytosis, solitary plasmacytoma, chordoma, pituitary adenoma, leiomyosarcoma, fungal infection, and mucocele which appeared primarily in sphenoid sinus are discussed along with their imaging features and pathological findings.
Conclusion:
Multitude of different pathologies can occur in sphenoid sinus. Detailed preoperative imaging is very helpful, but transnasal biopsy and histological study are required often for definitive diagnosis. The possible advantages of early diagnosis before spread of pathology for prognosis cannot be overemphasized.
Keywords
Chordoma
Langerhans cell histiocytosis
leiomyosarcoma
mucocele
pituitary adenoma
plasmacytoma
sphenoid sinus
INTRODUCTION
As more and more intracranial lesions are approached through the endoscopic transnasal route, neurosurgeons nowadays come across a lot of lesions in the sphenoid sinus with or without extension to intracranial cavity. We hereby describe our experience with few of the lesions which were in the sphenoid sinus. Isolated sphenoid sinus lesions are uncommon, accounting for 1%–2.7% of all paranasal sinuses lesions.[1] They usually present with nonspecific symptoms and are inaccessible by physical examination. The clinical presentation involves vague headaches and may be associated with purulent rhinorrhea, retropharyngeal drip, nasal obstruction, abnormal vision, and nerve deficits.[2]
The sphenoid sinus is anatomically in close relationship with the brain, meninges, optic nerve, internal carotid artery (ICA), cavernous sinus, and cranial nerve (oculomotor, trochlear, ophthalmic, and maxillary branches of the trigeminal nerve and abducens).[3] Delayed diagnosis and treatment can result in serious complications. Conventional radiographs do not allow a good view of the sphenoid sinus due to its location in the central skull base. Due its deep position, imaging technologies such as computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic biopsy play a significant role in understanding different pathologies affecting this region. The aim of this study is to demonstrate different pathologies which can affect the sphenoid sinus and elucidate the findings.
CASE ILLUSTRATIONS
-
A 12-year-old male child had headache for 2 months and diplopia for 15 days. Child had right 6th nerve paresis. CT showed a well-defined homogenously enhancing sphenoid sinus lesion. Extending superiorly and on both sides. MRI showed T1-hypo-isointemse, T2-hyper-isointense, and heterogeneously enhancing mass in sphenoid sinus. Patient underwent endoscopic transnasal decompression of lesion. Lesion was grayish white granular with moderate vascularity. Histopathology showed polymorphous cellular lesion with multiple conglomerate confluent histiocytic cells, with giant cells and mixed inflammatory cells. Hemorrhage and necrosis were noted. Histiocytes had a moderate amount of cytoplasm with oval nucleus and central constriction and groves. Immunohistochemistry (IHC) showed CD1a positive with MIB-1 labeling index of 10%–12% [Figure 1]. Diagnosis of Langerhans cell histiocytosis (LCH) was made. Patient was conservatively managed, and the child is asymptomatic at 18 months follow-up
-
A 43-year-old male had headache for 1 year, double vision for 8 months, and diminution of vision in both eyes for 1 month. He was on antiretroviral therapy for human immunodeficiency virus (HIV) infection since 3 years. CD4 count was 320 cells/mm3. He was previously treated for pulmonary tuberculosis 1 year back. The visual acuity was 6/18 bilaterally with left lateral rectus palsy. CT scan showed a sphenoid-sellar lesion. MRI showed well-defined lobulated homogeneous lesion involving the sphenoid sinus, sella, and clivus. The lesion was homogeneously isointense to gray matter on T2-weighted image (T2WI) and hyperintense on T2-weighted image (T1WI) without any diffusion restriction. There was no evidence of blooming on gradient images. Strong homogeneous postcontrast enhancement was noted. The lesion was seen invading bilateral cavernous sinuses. Patient underwent endoscopic transnasal decompression of the lesion. Histopathology showed cellular lesion with monomorphic small to medium cells in sheets, infiltrating bone which was strongly positive for lambda light chain and few cells for kappa chain, negative for cytokeratin, chromogranin, and pituitary hormones [Figure 2]. Features were suggestive of plasmacytoma. Systemic workup for myeloma was negative. Patient underwent radiotherapy, and MRI performed 1 year later showed no recurrence
-
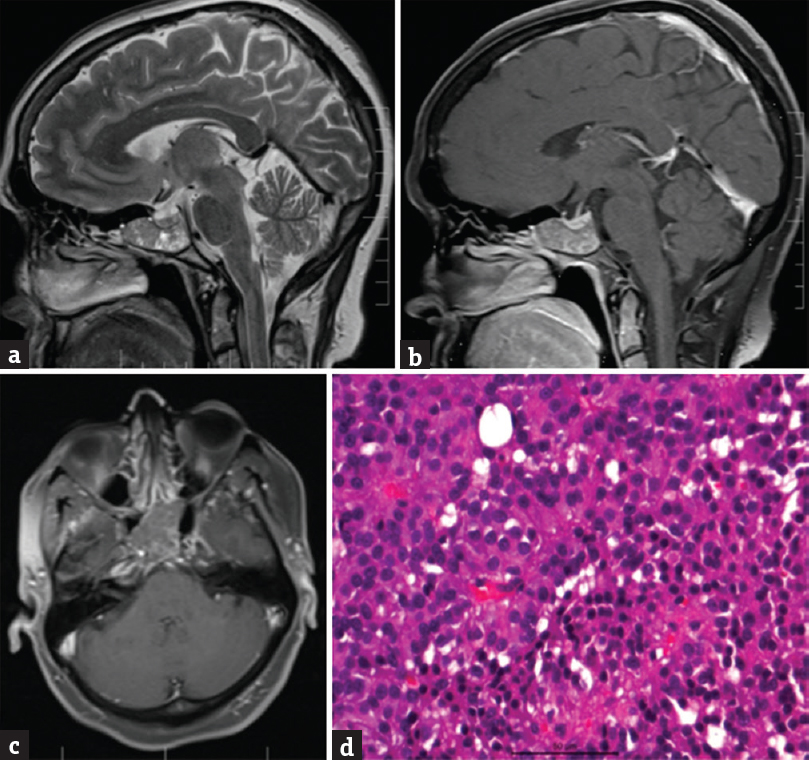
A 41-year-old male had headache and blurring of vision for 1 year, which increased in last 10 days. There was no perception of light in the left eye, and vision in the right eye was 6/9. CT showed well-defined sphenoid sinus lesion with sellar and suprasellar extension. MRI showed an expansile mass lesion arising from the sphenoid sinus expanding the sinus uniformly. Pituitary was seen separately and displaced superiorly. Lesion showed hypointense signal on T1WI, hyperintense on T2WI with scattered areas of microbleeds within. Diffusion-weighted imaging showed predominantly facilitated diffusion. On postcontrast study, variegated pattern of enhancement was seen within the lesion. Optic nerves appeared to be compressed at the optic canal level with superior displacement of the optic chiasm. Bilateral ICA was splayed by the mass lesion without any luminal narrowing. Patient underwent endoscopic transnasal transsphenoidal decompression of soft suckable vascular lesion inside sphenoid sinus. Histopathology showed a cellular neoplasm with prominent myxoid stroma, small epithelial-like dispersed cells with eosinophilic cytoplasm arranged in sheets, and trabeculae, interspersed with characteristic vacuolated physaliphorous cells [Figure 3]. Impression was chordoma. MRI at 3 months after surgery showed small residue for which radiotherapy was given
-
A 52-year-old male had headache for 5 years, and nasal bleeding for 1 month. He is a known case of Parkinson's disease on treatment. Although his visual acuity was normal, he had right temporal field defect. MRI was suggestive of primary sphenoid sinus lesion, with sellar extension. Patient underwent transnasal decompression of soft suckable vascular lesion. There was no bony defect seen in the sellar floor [Figure 4]. Histopathology was suggestive of ectopic pituitary adenoma. He underwent radiotherapy for residual tumor. MRI performed at 7 months after surgery showed very small residual lesion, which was managed conservatively
-
A 53-year-old male had headache for 8 months, decreased vision in the right eye, and numbness of face for 1 month. Visual acuity was limited to perception of light. MRI showed a sphenoid sinus lesion T1-isointense, T2-hypointense, and well enhancing on contrast. CT scan showed lesion extending till posterior nasal cavity with bone erosion. Patient underwent endoscopic transnasal decompression of lesion which was seen growing predominantly posterior and inferior, occluding choana superiorly till cranial base and nasal cavity. Lesion was firm, moderately vascular with cartilaginous feel. Dura was not involved. Histopathology of lesion showed variably cellular lesion composed of fragments of interlacing and whorled fascicles of spindle cells having oval flat ended nuclei and moderate eosinophilic cytoplasm. There were significant anisonucleosis and scattered mitosis (2–3/10 hpf) and evidence of bone invasion. IHC showed tumor cells to be strongly positive for vimentin and smooth muscle actin, negative for desmin and S-100. MIB-1 labeling was 8%–10% [Figure 5]. Features were suggestive of leiomyosarcoma (LMS). Patient was referred to oncologist for adjuvant therapy and is not available for follow-up
-
A 48-year-old diabetic female had double vision for 2 days. She had isolated left 6th nerve paresis. MRI showed a T1-hyperintense, T2-hypointense right-sided sphenoid sinus lesion, which was showing peripheral contrast enhancement. Patient underwent transsphenoidal biopsy. Histopathological examination revealed fungal ball with admixture of broad aseptate fungi with thin septate acute angled hyphae with chains of spores and conidia indicative of Aspergillus. Some hyphae were pigmented. There were no eosinophilic infiltrate or Charcot-Leyden crystals [Figure 6]. Final impression was combined zygomycosis and Aspergillus infection without any host response. Patient was put on voriconazole and was asymptomatic at 13 months follow-up
-
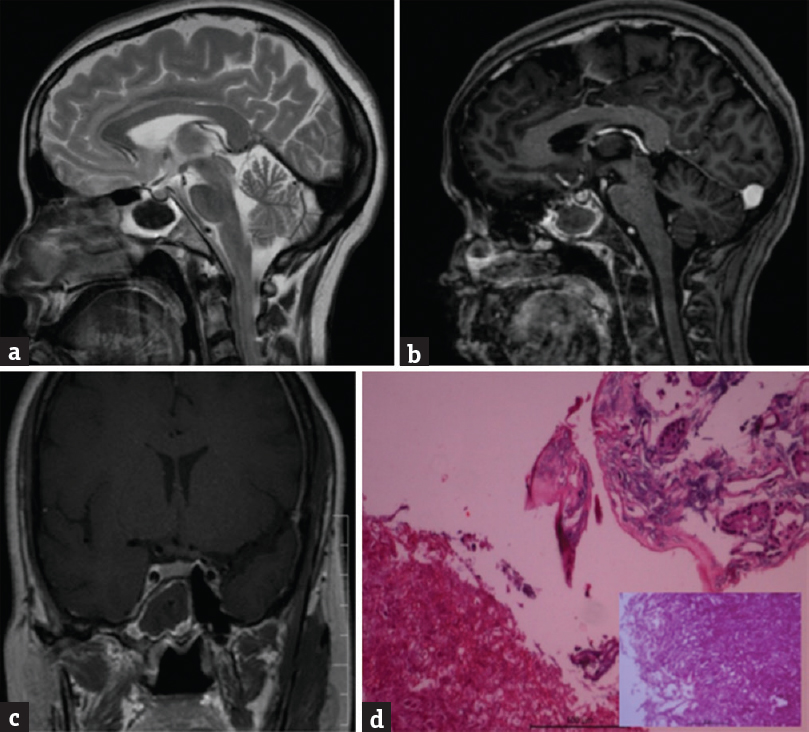
A 61-year-old male presented with progressive visual deterioration in the left eye, and ptosis for 1 month. He was previously operated for a mucocele about 5 years back. Patient underwent transnasal resection of lesion [Figure 7]. Diagnosis was sphenoid sinus mucocele.
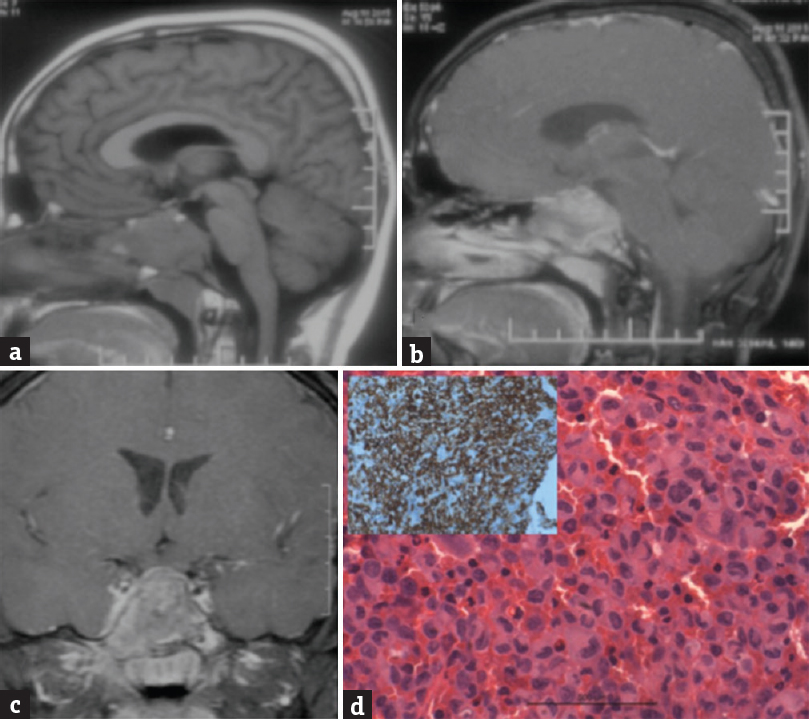
- (a) Magnetic resonance imaging T1-weighted image sagittal view showing an isointense mass lesion in the sphenoid sinus, (b) the lesion is enhancing on contrast on gadolinium contrast sagittal view, and (c) coronal view. (d) Shows a microphotograph with clusters of histiocytoid cells with a convoluted, indented nucleus admixed with giant cells and mixed inflammatory cells (H and E, ×400). Inset showing the tumor cells positive for CD1a immunostain (×400) suggestive of Langerhans cell histiocytosis

- (a) Showed a T1-weighted sagittal view showing an isointense mass lesion located in the sphenoid sinus with (b) showing postgadolinium contrast enhancement of the lesion in T1-weighted sagittal images and (c) coronal images. (d) Microphotograph showing respiratory mucosa (*) with a submucosal cellular infiltrating neoplasm (#) which is infiltrating the bone ($) as well (H and E, ×100). Inset shows the tumor composed of plasmacytoid cells (H and E, ×200) suggestive of plasmacytoma

- (a) Shows a T2-weighted sagittal magnetic resonance imaging with a hyperintense mass lesion located in the sphenoid sinus which is enlarged. (b) Shows the lesion in not enhancing on T1-weighted postgadolinium contrast compared to (c) axial T1-weighted image with a iso-hypointense mass located in the sphenoid sinus. (d) Microphotograph showing a myxoid neoplasm with diffusely scattered polyhedral cells having a vacuolated bubbly cytoplasm (Physaliphorous cells) in a highly myxoid stroma (H and E, ×400) suggestive of chordoma
- (a) Shows a T2 weighted sagittal magnetic resonance imaging image with a heterogeneously iso-hyperintense mass located in the sphenoid sinus, (b) the lesion is enhancing heterogeneously on postgadolinium contrast sagittal magnetic resonance imaging and (c) axial magnetic resonance imaging. (d) Microphotograph showing the neoplasm composed of closely packed acinar clusters of uniform round to polyhedral neuroendocrine cells (H and E, ×400) suggestive of pituitary adenoma
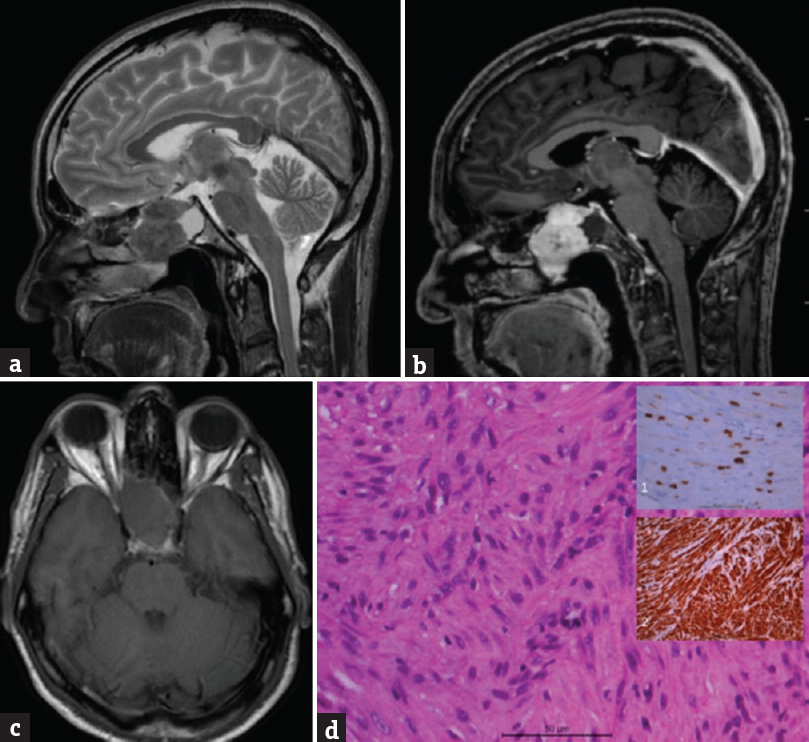
- (a) Showing a T2-weighted sagittal view with a heterogeneously iso-hypointense mass lesion located in the sphenoid sinus. (b) Shows heterogonous enhancement of lesion after gadolinium administration, (c) lesion is isointense on plain T1-weighted images. (d) Microphotographs showing a cellular spindle cell tumor with interlacing fascicles; inset (1) showing the increased MIB-1 labeling and inset (2) showing positive staining for smooth muscle actin. Vimentin positive but negative for S100, desmin, creatine kinase, and CD99 suggestive of leiomyosarcoma
- (a) Shows a T2-weighted sagittal magnetic resonance imaging with a hypointense mass lesion located inside the sphenoid sinus. (b) The lesion is not enhancing peripherally on postgadolinium T-weighted images (c) and in coronal images. (d) Microphotograph showing ulcerated mucosa with a colony of fungal hyphae morphologically resembling Aspergillus (H and E, ×100) inset shows the fungal colony with slender septate and branching hyphae (H and E, ×400)

- (a) Shows a T2-weighted sagittal magnetic resonance imaging with a hyperintense mass lesion in the sphenoid sinus with enlargement of sphenoid sinus. (b) The lesion is hyperintense on plain T1-weighted images and (c) not enhancing on postgadolinium injection axial and (d) coronal imagesab
DISCUSSION
The sphenoid sinus has often been neglected because of its isolated location and difficult access. The deep position of the sphenoid sinus prevents and hinders early diagnosis of pathologies in that location.[4] Delayed diagnosis and treatment can result in serious complications due to proximity of different vital structures. Imaging technologies such as CT and MRI along with the endoscopic surgical techniques have revolutionized the treatment strategies for lesions involving sphenoid sinus.
As more and more neurosurgical procedures are done through endoscopic transsphenoidal route, there is an increase in the number of sphenoid sinus lesions managed by neurosurgeons. Isolated sphenoid sinus lesions usually present with headache, followed by ophthalmological and nasal symptoms.[5] Of these lesions, 72% are inflammatory, 16% are neoplastic, and about 12% are because of other causes such as cerebrospinal fluid leak and fibrous dysplasia.[6] Although CT is investigation of choice, it is difficult to distinguish tumor from soft tissue swelling and secretions. MRI is thought to be complementary, but bony septae and malignant osseous lesions are poorly distinguished. Cases seen in neurosurgery are commonly evaluated by MRI. We presented imaging and histopathological findings of seven cases of isolated sphenoid sinus lesion with different diagnosis. In the above-illustrated cases, only lesions involving the sphenoid sinus were included. Patients were evaluated by objective ear, nose, and throat examination followed by imaging using MRI and a few cases with a CT as well. Isolated sphenoiditis due to infection is a common cause of such lesions, and hence a course of antibiotics for 3 weeks was given in cases where there was no suspicion about some other lesion. Such lesions were not included. We now discuss each pathological entity seen in sphenoid sinus.
LCH is a rare disorder with excessive proliferation of pathologic Langerhans cells. It is mainly seen in children and adolescents, but may occur in any age group.[7] It is commonly found in males, with a ratio of 2.5:1. The etiology of LCH is not well defined, and there is a lack of understanding with inconsistencies in the literature.[8] LCH is seen as hyperintense lesion on T2 and hypointense on T1-weighted MRI. LCH occurring in sphenoid sinus is very rare, and only a few cases have been described, especially involving neighboring structures.[91011] When involving only skull base, various modalities such as direct radiotherapy, only biopsy, biopsy and radiotherapy, or excision and radiotherapy have been reported with comparable efficacy. Our reported case was a child; hence he underwent decompression of lesion followed by observation.
Plasmacytomas are localized plasma cell tumors which can occur either in tissues (extramedullary) or in bones (solitary bone plasmacytomas).[12] Intracranial plasmacytomas usually present in the skull base and arise from the bones or from the soft tissue of the nasal cavity and paranasal sinuses.[13] Extramedullary plasmacytomas constitute approximately 3% of all neoplasms originating from plasma cells. They generally display a destructive course.[14] Till date, only twenty cases of sphenoid sinus plasmacytomas have been reported.[14] Our reported case was HIV positive leading to possibility of fungal, which was ruled out by IHC. There was no systemic involvement, and diagnosis of isolated sphenoid plasmacytoma was confirmed. Although the outcome of solitary plasmacytoma is variable, our patient was recurrence free after adjuvant therapy at 12 months follow-up.
Chordomas are midline tumors of the central nervous system, which arise from remnants of the primitive notochord where the heterotopic rests are usually situated extradurally within the bones of the axial skeleton. They are equally distributed in the skull base (32%), mobile spine (32.8%), and sacrum (29.2%), and of all intracranial tumors, skull base account for only 0.1%–0.2% of all chordomas.[15] Although clival chordoma is a well-known entity, our case was unique as it was predominantly intrasphenoidal leading to possibility of alternate diagnosis. There are only a few cases where chordoma has been reported to be arising predominantly from sphenoid sinus.[16171819] Although the role of radiotherapy is controversial, we still chose to treat this patient for residual lesion.
Ectopic sphenoid sinus pituitary adenoma by definition is the tumors centered within the sphenoid sinus and demonstrated, by imaging studies or intraoperative examination, a normal sella turcica without a concurrent pituitary adenoma.[20] Ectopic pituitary adenomas are derived from embryologic remnants along the path of migration of Rathke's pouch, and more than fifty ectopic pituitary adenomas have been reported.[21] Ectopic pituitary adenomas may be found in the sphenoid sinus region, clivus, parapharyngeal space, nasal cavity and nasopharynx, hypothalamus, third ventricle, and in the suprasellar locations.[22] About two-thirds are hormonally active adenomas.[23] Our reported case was a nonfunctional pituitary adenoma which was managed surgically and with radiotherapy for the residual lesion.
LMS is a disease of the gastrointestinal and genitourinary tracts. It has been associated with a history of irradiation, retinoblastoma, chemotherapy, HIV, and AIDS. Approximately, fifty cases of primary sinonasal LMS have been reported so far, but only one case is described where LMS originated in sphenoid sinus.[24] LMS arises most frequently from smooth muscle cells within the walls of blood vessels but may also come from undifferentiated mesenchymal cells. Pathologically, the tumors may range from extremely well differentiated to anaplastic. Prognosis for primary LMS remains poor, with overall 5- and 10-year survival rates of 20% and 6%, respectively.[25] Approximately 8%–56% of patients will develop metastatic disease.[26] LMS of the nasal cavity and paranasal sinuses has a more favorable clinical course, with small tumors limited to the nasal cavity having the better prognosis.[27] Wide local excision is treatment of choice. Tumor size at the time of diagnosis and complete excision appear to be the most significant prognostic factors. Adjuvant chemotherapy with or without radiation therapy is recommended.[24] We referred our patient for adjuvant therapy to oncologist.
Fungal sinusitis is classified into allergic, chronic noninvasive (fungus ball), chronic invasive, granulomatous invasive, and acute fulminant invasive fungal sinusitis based on histological features according to the diagnostic criteria of deShazo et al.[2829] The rarest occurrence of invasive fungal sinusitis is seen in the sphenoid sinus.[30] Clinical manifestations are dependent on the immune status of patients given the ubiquitous nature of these organisms. The presence of neurological findings due to intracranial complication should be investigated immediately. Although bone destruction is one of the radiologic findings of invasive fungal sinusitis, mucor extension along the blood vessels can with intact bony sinus walls.[31] MRI is superior modality of investigation when intracranial symptoms are present as it reveals soft tissue involvement.[32] Even though imaging can give a clue about fungal sinusitis, the microbiological identification of fungus is of paramount in the management of the infection[33] and differentiation from allergic fungal rhinosinusitis. Advanced invasive fungal infection of the sphenoid sinus carries significant mortality. Hence, early diagnosis and appropriate treatment are crucial.[34] Although noninvasive fungal sphenoid sinusitis rarely presents with serious complications. Diplopia and transient vision loss have been reported in up to 3% of cases.[30] Three most common fungal infections in the nasal sinuses are the opportunistic genera of Aspergillus, Mucor, and Candida, of which Aspergillus is the most prevalent and Mucor is most invasive.[35] It is considered that surgical treatment should aim more at radically removing the mycotic infected lesion, rather than draining it though there is no significant difference in outcome even if drainage is done, especially in invasive fungal sinusitis.[36] Postoperative progression of disease even after antimycotic therapy may lead to fatal outcomes.[37] Our reported case was of primary focal fungal ball with combined zygomycosis and Aspergillus infection without invasion, successfully treated with decompression followed by antifungal therapy.
Mucocele is defined as the accumulation and retention of mucoid secretion within a paranasal sinus, leading to thinning and distension and erosion of one or several of bony walls.[38] While primary mucoceles occur as retention cysts of the mucous glands of sinus epithelium, secondary mucoceles arise from the obstruction of the sinus ostium. Sphenoid mucocele comprises 1%–2% of all mucoceles.[39] Less than 150 cases of sphenoid sinus mucoceles have been described in the literature.[4041] Headache is the most common symptom, and visual disturbance is the second most common symptom.[38] Usually, the cranial nerves involvement brings the patient to the physician. Our reported patient had 2nd and 3rd nerve involvement which improved after surgery, but the exact cause of recurrent mucocele could not be found out.
Other common pathologies such as meningocele/meningoencephalocele,[42] osteomas,[43] lymphoma,[44] metastasis,[45] other cancers,[4647] and rare pathologies such as inverted papillomas,[48] epidermoids,[49] melanoma,[50] myxoma,[51] esthesioneuroblastoma,[52] trigeminal schwannoma,[53] and many others have been described. The sphenoid sinus may be the starting point for primary malignant tumors of different histological types.[54] Although CT and MRI can help suspect a malignancy, only transnasal biopsy and histological study allow the diagnosis. The possible advantages of early diagnosis before spread of pathology for prognosis cannot be overemphasized.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Isolated sphenoid sinus disease: An analysis of 122 cases. Ann Otol Rhinol Laryngol. 2002;111:323-7.
- [Google Scholar]
- The clinical behavior of isolated sphenoid sinusitis. Otolaryngol Head Neck Surg. 2007;136:610-5.
- [Google Scholar]
- Infectious and neoplastic diseases of the sphenoid sinus – A report of 10 cases. Rhinology. 2002;40:34-40.
- [Google Scholar]
- Isolated sphenoid sinus disease: An analysis of 132 cases. Laryngoscope. 1997;107(12 Pt 1):1590-5.
- [Google Scholar]
- Isolated sphenoid sinus disease: Etiology and management. Otolaryngol Head Neck Surg. 2005;133:544-50.
- [Google Scholar]
- Langerhans cell histiocytosis of spine: A comparative study of clinical, imaging features, and diagnosis in children, adolescents, and adults. Spine J. 2013;13:1108-17.
- [Google Scholar]
- Langerhans cell histiocytosis: Diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278-90.
- [Google Scholar]
- Solitary langerhans-cell histiocytosis of the clivus and sphenoid sinus with parasellar and petrous extensions: Case report and a review of literature. Surg Neurol. 2004;62:447-54.
- [Google Scholar]
- Langerhans cell histiocytosis involving the sphenoid sinus and superior orbital fissure. AJNR Am J Neuroradiol. 1995;16(4 Suppl):964-7.
- [Google Scholar]
- Langerhans cell histiocytosis of the sphenoid sinus: A case report. Turk J Pediatr. 2010;52:548-51.
- [Google Scholar]
- Cranial nerve palsy in multiple myeloma and solitary plasmacytoma. Asia Pac J Clin Oncol. 2010;6:251-5.
- [Google Scholar]
- A case of extramedullary plasmacytoma in the sphenoid sinus with unilateral loss of vision. J Craniomaxillofac Surg. 2013;41:140-3.
- [Google Scholar]
- Chordoma: Incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1-11.
- [Google Scholar]
- Intracranial chordoma arising from the sphenoid sinus. J Iowa Med Soc. 1967;57:911-5.
- [Google Scholar]
- Chordoma originated from sphenoid sinus, encroach on sella, metasella and clivus: One case report. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:207-8.
- [Google Scholar]
- A case of primary chordoma of the sphenoidal sinus. J Laryngol Otol. 1961;75:429-32.
- [Google Scholar]
- Chondroid chordoma of the sphenoid sinus – A rare tumour. Online J Otolaryngol. 2014;4:101-14.
- [Google Scholar]
- Ectopic sphenoid sinus pituitary adenoma (ESSPA) with normal anterior pituitary gland: A clinicopathologic and immunophenotypic study of 32 cases with a comprehensive review of the english literature. Head Neck Pathol. 2012;6:75-100.
- [Google Scholar]
- Right ectopic sphenoid sinus pituitary adenoma. Braz J Otorhinolaryngol. 2014;80:451-2.
- [Google Scholar]
- Suprasellar adrenocorticotropic hormone-secreting ectopic pituitary adenoma: Case report and literature review. Neurosurgery. 2002;50:618-25.
- [Google Scholar]
- Extracranial thyroid-stimulating hormone-secreting ectopic pituitary adenoma of the nasopharynx. Otolaryngol Head Neck Surg. 2005;133:453-4.
- [Google Scholar]
- Primary leiomyosarcoma of the sphenoid sinus. Arch Otolaryngol Head Neck Surg. 2009;135:949-52.
- [Google Scholar]
- Sinonasal leiomyosarcoma: Review of literature and case report. Laryngoscope. 2005;115:2242-8.
- [Google Scholar]
- Leiomyosarcoma of the head and neck: A clinicopathological study. Histopathology. 2002;40:518-25.
- [Google Scholar]
- Sinonasal smooth muscle cell tumors: A clinicopathologic and immunohistochemical analysis of 12 cases with emphasis on the low-grade end of the spectrum. Arch Pathol Lab Med. 2003;127:297-304.
- [Google Scholar]
- A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181-8.
- [Google Scholar]
- Temporal lobe abscess in a patient with isolated sphenoiditis. Allergy Rhinol (Providence). 2011;2:40-2.
- [Google Scholar]
- Imaging features of invasive and noninvasive fungal sinusitis: A review. Radiographics. 2007;27:1283-96.
- [Google Scholar]
- Invasive fungal sinusitis of the sphenoid sinus. Clin Exp Otorhinolaryngol. 2014;7:181-7.
- [Google Scholar]
- Invasive fungal rhinosinusitis in immunocompromised patients. Rhinology. 2004;42:141-4.
- [Google Scholar]
- Primary aspergillosis of the sphenoid sinus with pituitary invasion – A rare differential diagnosis of sellar lesions. Acta Neurochir (Wien). 2006;148:1085-90.
- [Google Scholar]
- Successful treatment of an invasive aspergillosis of the skull base and paranasal sinuses with liposomal amphotericin B and itraconazole. Ann Otol Rhinol Laryngol. 1999;108:205-7.
- [Google Scholar]
- Mucocele of the sphenoid sinus: A rare cause of reversible 3rd nerve palsy. Ann Indian Acad Neurol. 2012;15:158-60.
- [Google Scholar]
- Sphenoid sinus mucocoele presenting with isolated oculomotor nerve palsy. J Laryngol Otol. 1997;111:471-3.
- [Google Scholar]
- Paranasal sinus mucoceles: About 32 cases. Rev Stomatol Chir Maxillofac Chir Orale. 2016;117:11-4.
- [Google Scholar]
- Clinical analysis of patients with sphenoid sinus mucocele and literature review. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:1850-2.
- [Google Scholar]
- Lateral sphenoid sinus recess cerebrospinal fluid leak: A case series. Eur Arch Otorhinolaryngol. 2014;271:2587-94.
- [Google Scholar]
- The endoscopic transnasal paraseptal approach to a sphenoid sinus osteoma: Case report and literature review. Ear Nose Throat J. 2013;92:E7-10.
- [Google Scholar]
- Non-Hodgkin's lymphoma of the sphenoid sinus presenting as isolated oculomotor nerve palsy. World J Surg Oncol. 2007;5:86.
- [Google Scholar]
- Gastric metastasis from sphenoid sinus melanoma: A case report. Oncol Lett. 2015;9:609-13.
- [Google Scholar]
- Papillary carcinoma of the sphenoid sinus associated with sphenoid sinus abscess presenting as cavernous sinus syndrome. A case report. J Clin Neuroophthalmol. 1990;10:18-20.
- [Google Scholar]
- Primary cancers of sphenoidal sinus. J Fr Otorhinolaryngol Audiophonol Chir Maxillofac. 1968;17:45-54.
- [Google Scholar]
- Isolated inverted papilloma of the sphenoid sinus. J Chin Med Assoc. 2010;73:503-5.
- [Google Scholar]
- Epidermoid cyst of the sphenoid sinus with extension into the sella turcica presenting as pituitary apoplexy: Case report. Surg Neurol. 2005;63:394-7.
- [Google Scholar]
- Primary melanoma of the sphenoid sinus presenting with a third cranial nerve palsy. J Neuroophthalmol. 2005;25:289-92.
- [Google Scholar]
- Sphenoid sinus myxoma: Case report and literature review. Ochsner J. 2008;8:166-71.
- [Google Scholar]
- Fine needle aspiration cytology of primary sphenoid sinus esthesioneuroblastoma metastatic to the skin. Avicenna J Med. 2012;2:15-8.
- [Google Scholar]
- Trigeminal schwannoma of the sphenoid sinus – Report of a rare entity. Br J Neurosurg. 2014;28:281-3.
- [Google Scholar]






