Translate this page into:
Incidence, histopathology, and surgical outcome of tumors of spinal cord, nerve roots, meninges, and vertebral column - Data based on single institutional (Sher-i-Kashmir Institute of Medical Sciences) experience
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
In the absence of a community-based study on the spinal tumors in the Valley, medical records of the only Regional Neurosurgical Center are available.
Aim:
The aim of this study is to establish a hospital-based regional epidemiology of spinal tumors in the Valley since the data are derived from a single institution.
Materials and Methods:
A retrospective analysis of 531 malignant and nonmalignant tumors of spinal cord, its coverings and vertebrae, which were managed in a Regional Neurosurgical Center under a standard and uniform medical-protocol over 30-year period from 1983 to 2014.
Results:
The hospital-based incidence for all spinal tumors was 0.24/100,000 persons per year. The malignant spinal cord and vertebral tumors comprised 32.58% (173/531) of all tumors, and benign spinal cord and vertebral tumors comprised 67.42% (358/531). The extradural–intradural tumors such as metastatic lesions and primary malignant vertebral tumors were on rise with 16.38% (87/531) cases. The children below 18 years were 5.46% (29/531), of which 55.17% (16/29) were below 9 years. The most common primary bone malignancy was multiple myeloma (54.54% =12/22). Histopathologically, the most common metastatic deposit in the spinal canal was non-Hodgkin's lymphoma (24.61% =16/65). A mortality of 3.20% (17/531) was noted. Recurrences were noted in 4.90% (26/531), and adjuvant therapies were given to 16.38% (87/531) patients.
Conclusion:
The malignant spinal cord and vertebral tumors, especially metastatic deposits, are on rise in elderly population. The surgical outcome, in terms of recovery and spinal stability, of benign tumors, is comparatively better than malignant ones. The study reveals a low regional incidence (hospital-based) of spinal tumors.
Keywords
Histopathology
hospital-based incidence
spinal tumors
surgical outcomes
Introduction
The spinal cord, caudal tail-like extension of the brain with millions of neurons, ascending and descending tracts, nerve fibrils, vessels, cerebrospinal fluid (CSF) spaces, etc., is hanging by nerve roots, pial bands, and shrouded within delicate and fibrous meninges, ligaments, vascular network, and a long segmented strong bony cage called spinal column. The malignant and benign tumors of the spinal column, meninges, nerve roots, or the cord parenchyma itself may compress and damage components of the spinal cord to cause neural deficits at all ages. However, Duong et al. showed the age-adjusted incidence of nonmalignant primary spinal tumors as 0.76/lakh population in the USA and for the malignant spinal tumors, it has been only 0.22/lakh persons.[1] The hospital-based incidences of various tumors, in the occurrence of age, histological tumor type, and outcome, were revealed in the analysis of 531 tumors of the spinal cord, nerves, meninges, and bone, which were managed over a period of more than 30 years in the only tertiary Neurosurgical Centre available in this mountain-locked and snow-cold Northern Valley of Kashmir. This single hospital-based epidemiological study, in the absence of any such or community-based epidemiological study in the region, will hopefully serve the purpose of spinal tumor predictability in a given population and the approaches of management.
Materials and Methods
The Department of Neurosurgery conducted a retrospective study on the patients of spinal (cord, its roots, meninges, and vertebrae) tumors (from C1 to lower border of L1), surgically treated for more than 30 years from January 1983 to December 2014. All the patients were admitted after clinical assessment under a standard and medical protocol and subjected to complete basic hematochemical and radio-imaging evaluation. After preanesthetic check-up, patients were posted for the planned microsurgical procedure under C-arm control. The intraoperative ultrasonography was used when required. All the patients received an intraoperative infusion of bolus dose of methyl prednisolone succinate at the time of surgery and subsequently if required a maintenance dose for next 23 h. The histopathological examination of the biopsy specimen was carried out. The histopathology was the mainstay of final diagnosis. All the patients were categorized according to the anatomical site of the lesion in relation to the dura mater and the cord parenchyma such as intradural (ID), extradural (ED), intramedullary (IM), and extramedullary (EM). The anatomical spinal level of the lesion was ascertained by the clinical examination. The location and the nature of the lesion in the regions such as cervical, dorsal, or lumbar was ascertained by the radio-imaging and intraoperative use of C-arm (fluoroscopy imaging). The Nuick's classification of disability was used to measure the pre- and post-operative functional motor weakness in all the patients where Grade 0 was called as good recovery.[2] These were subdivided according to the histopathological analysis of the tumor tissue such as benign or malignant and the tissue of origin such as primary (local) and secondary (metastatic deposit from a primary in a distant organ). The lesions which overlaid the dura, adhered to penetrate it and grew intradurally were called ED/ID lesions whether primary from adjacent vertebrae or metastatic. The spinal radiology and imaging of the patients was divided (according to the era) in 4 groups such as (1) myelography (1983–1994), (2) myelo-computed tomography (CT) (1988–1994), (3) CT-scan and contrast-enhanced computed tomography CECT (1988–1994), and (4) magnetic resonance imaging (MRI) (1994 onward). However, MRI was the mainstay of the diagnosis in all patients after 1994 AD. The bone scan (99m Tc-MDP) was used, which is more sensitive than plain radiographs for detecting spinal metastases, in all suspected cases. The advantage of bone scan is the ability to screen the entire skeleton with a single image. The data were compiled, analyzed, and results drawn. The statistical law of variance was used wherever applicable.
Observations
The incidence
Presuming the population of this northern Province of India, as 7 million (J and K Census 2010–2011), observations revealed that the incidence of spinal cord and bone, primary and metastatic tumors in this Province is about 0.24/100,000 persons per year for all ages while for the children (18 years and below), it was lowest – 0.014/100,000 persons per year [Tables 1 and 2]. The malignant spinal cord and bone tumors were incidentally found in 0.08/100,000 person per year whereas benign tumors were in 0.17/100,000 persons per year. The incidence in men is 0.14/100,000 persons per year and in women 0.11/100,000 persons per year. The malignant spinal cord and bone tumors comprised 32.58% (173/531) of all tumors and benign spinal cord and bone tumors comprised 67.42% (358/531).
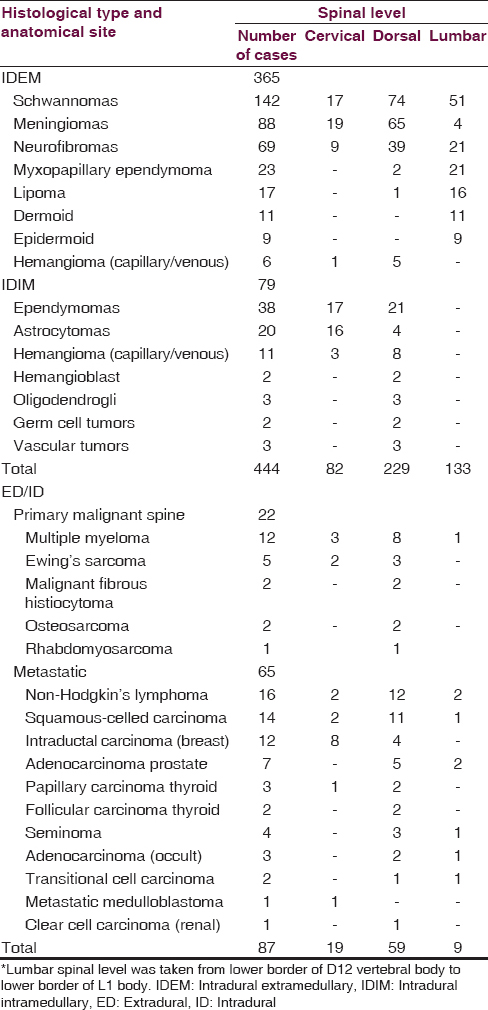
Age and sex
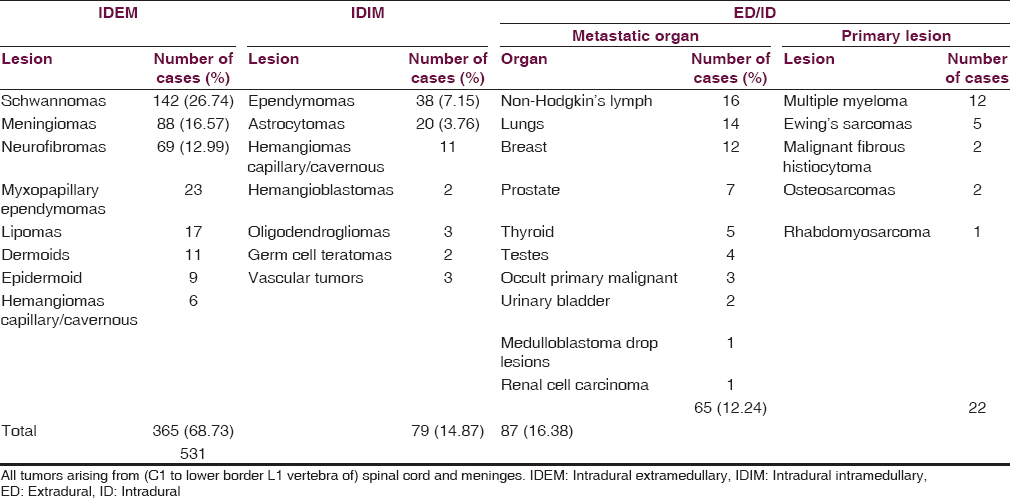
The analysis revealed that ED tumors such as metastatic lesions [Figure 1] and primary malignant bone tumors were on rise (16.38% =87/531), especially in elderly above 60 years of age 9.79% (=52/531) [Table 3]. The metastatic deposits alone accounted for 12.24% (65/531), most frequently from non-Hodgkin's lymphoma followed by squamous-celled carcinoma (2.63% =14/531) from the lungs and the intraductal carcinoma from breast (12/531 = 2.25%). The children below 18 years had 5.46% (29/531) tumors, in which most (3.01% =16/531) children were below 9 years of age [Figure 2]. A male preponderance (297/531 = 55.93%) for almost all ages was recorded with male:female ratio of 1.26:1.00. IDEM tumors (365/531 = 68.73%) were mostly found in fourth and fifth decades whereas IDIM tumors (14.87% =79/531) were seen in third and fourth decades and mostly in males.
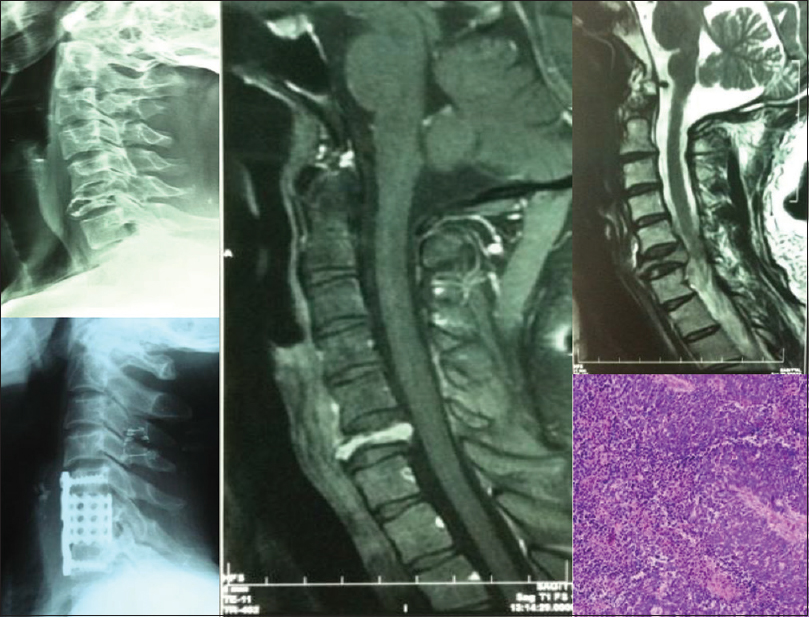
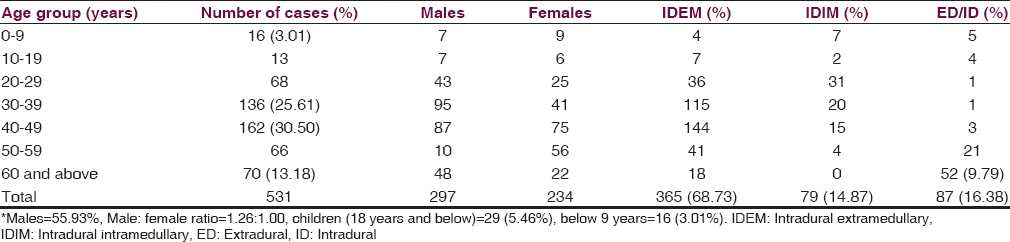
- Pre- and Post-operative X-rays, preoperative magnetic resonance imaging and histopathological microphotograph of a completely destroyed 6th cervical vertebral body in an elderly male, compressing spinal cord, due to metastatic deposits from carcinoma of lung
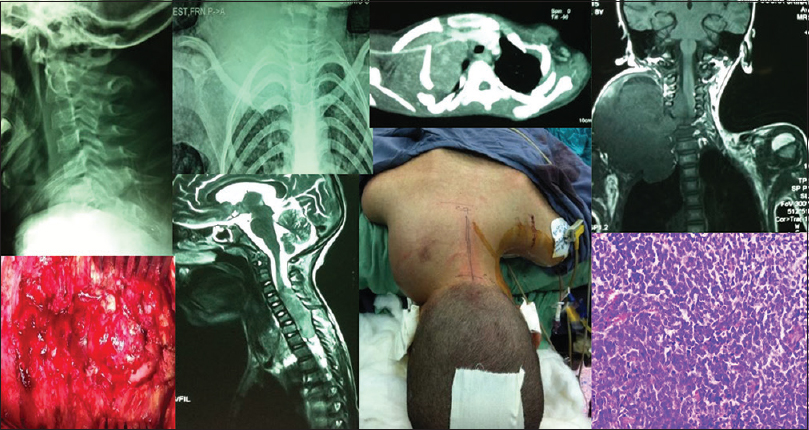
- A male child of 8 years with Ewing's sarcoma shows wide cervical spinal canal and tracheal shift to left on X-rays, while axial CT, coronal and sagittal magnetic resonance imaging chest, and cervical spine display intraspinal tumor extension from C3 to C7 and upper lobe of left lung. The clinical, intraoperative, and histopathological microphotograph (H and E, ×400) of tumor is shown
Level and site of origin
IDEM tumors occurred in 68.73% (365/531) patients and the most common tumor type, schwannoma [Figure 3], was found in 38.90% (142/365) patients followed by the meningiomas 24.10% (88/365) and neurofibromas 18.90% (69/365). The most common spinal level affected by all tumors was dorsal spine in 54.24% (288/531) patients. About 20.27% (74/365) schwannomas and 10.68% (39/365) neurofibromas manifested as dorsal cord compressive lesions. However, 17.80% (65/365) meningiomas occurred in dorsal level followed by 5.20% (19/365) in cervical cord [Figure 4].
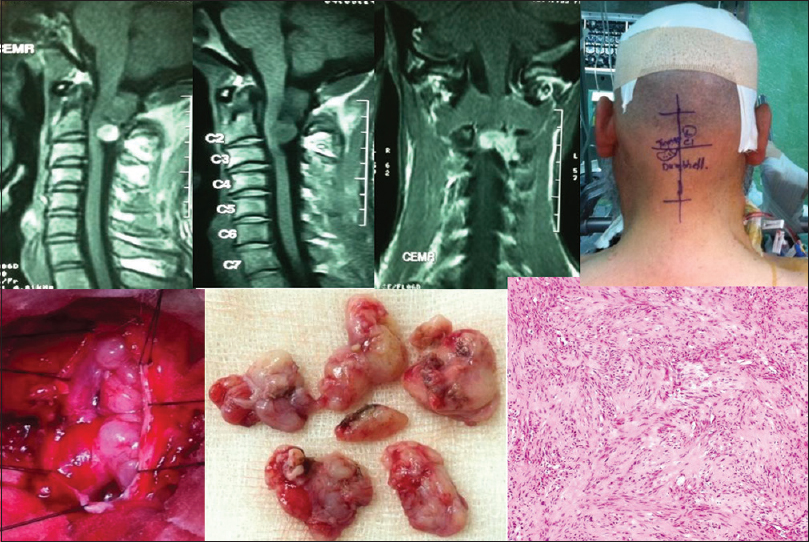
- The contrast MR images, intraoperative and specimen photographs and histopathological microphotograph (H and E, ×40) of a high cervical-cord (C1, 2) dumbbell schwannoma are shown
- Intradural extramedullary high cervical meningioma (left sided– at C2, 3, 4) with dural-tail extending up to C4 vertebral body– complete excision. A typical cervical myelogram, magnetic resonance imaging, and intraoperative photographs show Stretching of Left C2, 3, 4 nerve roots and pushed-cord. Low-magnification histological microphotograph of the tumor is displayed
Histopathological types
The other IDEM tumors such as myxopapillary ependymomas [Figure 5], lipomas, dermoids [Figure 6], epidermoids and capillary, or venous hemangiomas were found in 18.08% (66/365) patients and almost all of these occurred in first lumbar level except hemangiomas that mostly occupied dorsal ID space [Tables 1 and 2]. About 14.87% (79/531) IM tumors comprised 48.10% (38/79) ependymomas [Figure 7], equally distributed in dorsal and cervical spinal cord, followed by 25.31% (20/79) astrocytomas that mostly occupied cervical cord. The hemangiomas, oligodendrogliomas, vascular tumors, hemangioblastomas, and germ cell tumors were next common IDIM lesions and almost all originated from the dorsal spinal cord. The ED-ID lesions comprised more than half (50.28% = 87/173) of all the primary and secondary malignant bone and spinal cord tumors, contributing 25.28% (22/87) primary malignant bone tumors and 74.71% (65/87) metastatic deposits. Among primary malignancy of spinal column bone and soft tissue, the multiple myeloma accounted for 54.54% (12/22) patients, of which 2/3rd grew from dorsal spinal level and the rest 1/3rd from cervical and first lumbar level. The Ewing's sarcoma [Figure 2] was found in 22.72% (5/22) cases, which mostly occupied dorsal and cervical spinal canal. Among the rest of ED primary malignancy, the malignant fibrous histiocytoma, osteosarcoma, and a single case of rhabdomyosarcoma were concentrated in the dorsal spine. The most common metastatic deposit in the spinal canal was from non-Hodgkin's lymphoma (24.61% = 16/65) and that too mostly (75% =12/16) in the dorsal canal [Table 1]. The second common deposits were histologically squamous-celled carcinoma (21.53% =14/65) from the lungs [Figure 1] and mostly (78.57% =11/14) settled in the dorsal region. The intraductal carcinoma from breast (18.46% =12/65) was found 66.66% (8/12) in the cervical region and 33.33% (4/12) in the dorsal area. The metastasis from the adenocarcinoma of prostate, papillary and follicular carcinoma of thyroid, seminoma, transitional carcinoma of bladder, and clear cell carcinoma of kidney frequently precipitated in the dorsal spine. The only case of drop metastasis from the medulloblastoma in a child was located in the cervical spinal level [Table 1].
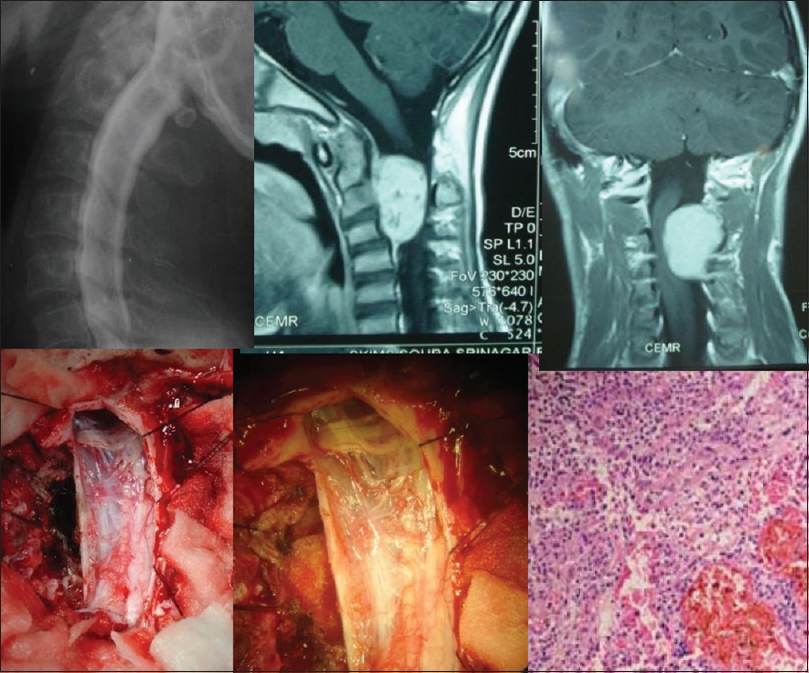
- A conventional myelogram, magnetic resonance imaging, intraoperative and specimen photographs of an exophytic-intramedullary myxopapillary ependymoma at D12, L1 level, with histological microphotograph (H and E, ×400) is shown

- The magnetic resonance imaging, intraoperative-microscopic, specimen and histological photographs of an intramedullary dorsal (D3) spinal cord dermoid. The microphotograph shows abundant cellular keratin, calcification and keratinized stratified squamous epithelium; H and E, ×10
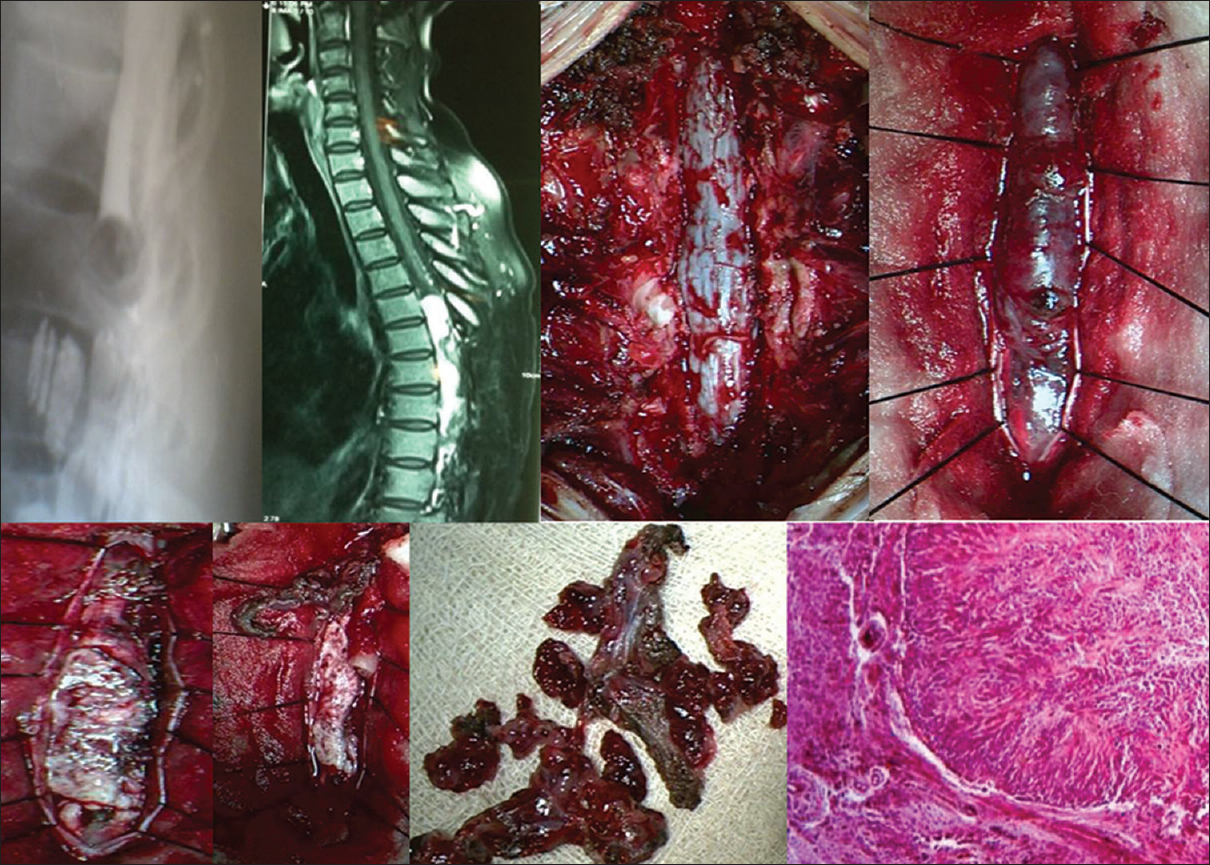
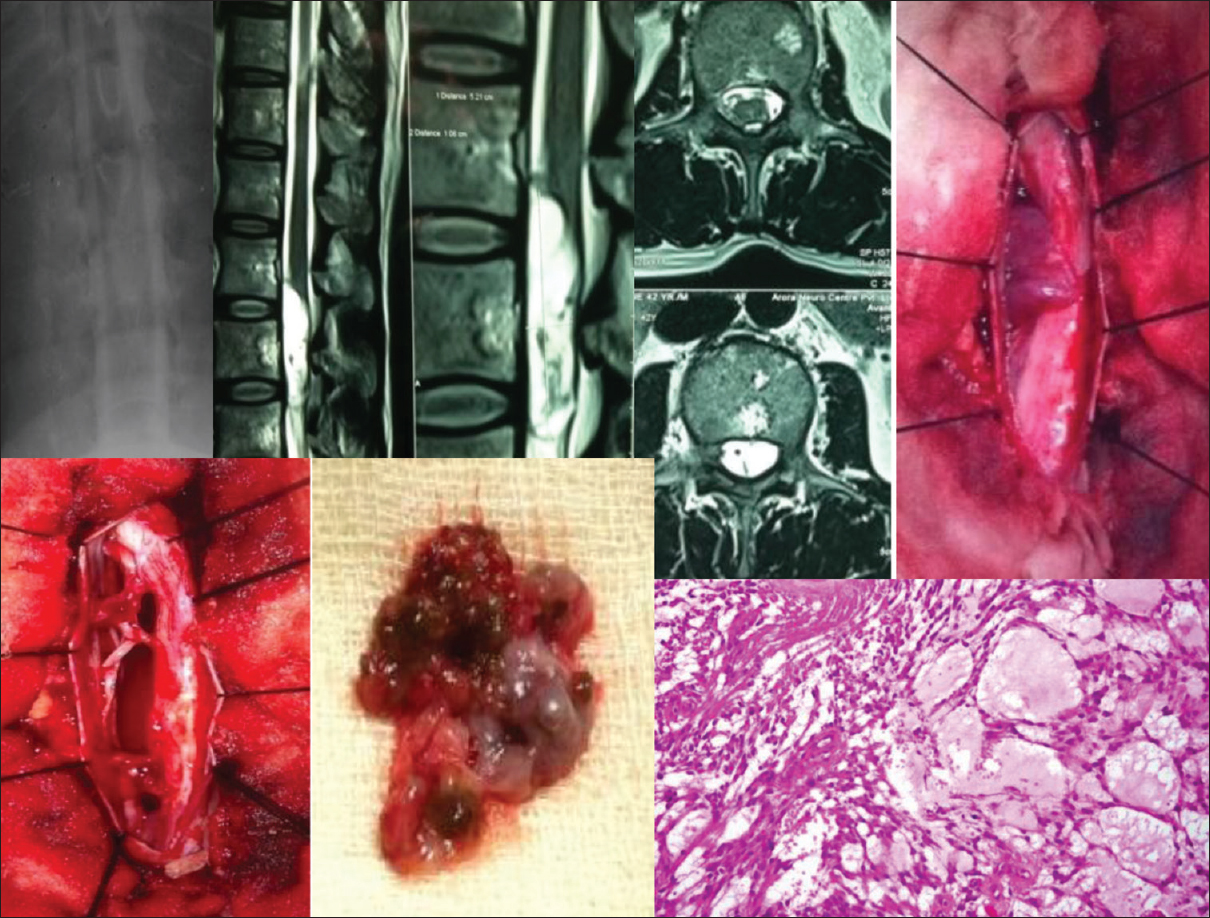
- Dorsal myelographic filling defect, Sagittal MR T1WI, Intraoperative and specimen photographs with histopathological microphotograph (H and E, low magnification) of a spinal intramedullary malignant tumor (Ependymoma) extending from D5 to D12 are displayed
Management and surgical outcome
MRI [Figures 1, 3, and 4] has been the mainstay of the diagnosis in 71.18% (378/531) patients after 1994 AD. The myelography [Figures 4, 5, and 7] was performed from 1983 to 1994 in 8.85% (47/531) patients and the Myelo-CT (1988–1994) in 19.96% (106/531) cases. The CT-scan plain [Figure 2] and CECT (1988–1994) spine was performed in 4.33% (23/531) spinal tumors. The bone scan (99m Tc-MDP) detected 86.20% (75/87) of all the metastatic and primary malignant spinal bone tumors. The microsurgical procedures were performed on all 531 patients under the control of C-arm, intraoperative ultrasonography, and infusion of methyl prednisolone succinate at and above lumbar one body level. The total surgical excision of tumor was carried out in 81.54% (433/531) patients, in which 1.88% (10/531) were ED-ID, 10.92% (58/531) IDIM, and 68.73% (365/531) were IDEM. ED-ID, IDEM, and IM tumors were operated upon by the posterior approach in the supine or lateral position with laminectomy or laminoplasty of the level of interest in cervical, dorsal, and dorsolumbar region. ED-ID lesions such as multiple myeloma, non-Hodgkin's lymphoma, and malignant fibrous histiocytoma were excised microscopically in total and subjected to postsurgical adjuvant radiochemotherapy. The schwannomas, neurofibromas, meningiomas, and myxopapillary ependymomas were excised in total or near total. The postoperative adjuvant radiotherapy was only advised for myxopapillary ependymoma patients to contain CSF metastasis. IM tumors such as ependymoma were subjected to the maximum surgical debulking whereas astrocytomas had multiple tumor site biopsies to decompress the cord parenchyma as well as to achieve the histopathological diagnosis for postoperative adjuvant radiotherapy. The multisite biopsies with decompression preserved the overall neural function of the patients and also maintained the anatomical integrity of the spinal cord. The posterior fusion with Harrington rods and transpedicular screw-fixation in the cervical and dorsal spine (including lumbar 1 and 2) was performed only if 3 or more than 3 laminae were removed, unilateral or bilateral lateral ½ facetectomy was done and laminectomy at cervicodorsal or dorsolumbar regions was performed to excise a tumor. However, hemilaminectomies were also performed in 11.50% (42/365) cases of IDEM. All cervical, dorsal, and dorsolumbar vertebral primary tumors, metastatic deposits, extensive IDEM tumors with vertebral invasion, and vascular tumors with collapse of vertebral bodies were approached by the anterior, lateral, anterolateral, and posterolateral approaches to perform a decompressive corpectomy. The anterior cervical and lateral clavico-sternotomy approaches were used to do a cervical corpectomy with a cage and plate fixation. The lateral position was used for the modified posterolateral rib resection/intercostal transthoracic intrapleural, modified posterolateral rib resection/intercostal transthoracic extrapleural retroperitoneal and anterolateral retroperitoneal approaches to fix dorsal and lumbar spine. Transaxillary (modified Sher-i-Kashmir Institute of Medical Sciences [SKIMS]) Approach was specifically used for decompressive corpectomy and instrumentation in the C7, D1 area. The materials for the fusion used were either autologous (iliac crest graft, fibular bone graft, rib graft, spinous process graft, etc.) or synthetic materials. The synthetic material of choice used, for the interbody fusion postdecompression of spinal segments involved by the aggressively invasive primary malignant (Ewing's tumor etc.,) and metastatic vertebral tumors, was methyl-methacrylate (bone cement). The primary malignant bone and metastatic deposits were treated with maximum debulking to achieve standard stability of the spine by instrumentation/fixation [Figure 1] and beneficial results from postoperative radiation therapy. All primary malignant and metastatic tumors were treated with adjuvant radiation and chemotherapy. About 76.08% (404/531) patients showed postoperative improvement in their neurodeficits whereas 27.68% (147/531) of these showed good recovery. However, 4.14% (22/531) worsened postoperatively and had moderate to severe disability. The mortality of 3.20% (17/531) was recorded and all of these patients were metastatic spines 26.15% (17/65). A total of 16.57% (88/531) patients developed various complications. The causes of mortality in 17 patients were surgical complications in 0.94% (5/531) and disease progression in 2.26% (12/531). All of these patients died within the postoperative follow-up period of 9 months. The complications occurred in 88 patients and these were hemorrhage and wound infection in 7.72% (41/531), CSF leak in 3.95% (21/531), spinal shock in 3.01% (16/531), meningitis in 1.32% (7/531), and acute respiratory insufficiency in 0.56% (3/531). The postsurgical recurrences and residual tumors were noted in 4.90% (26/531) patients within 9 months of postoperative follow-up. The adjuvant therapy in the form of radiotherapy and chemotherapy was given to 16.38% (87/531) patients within 1–3 months of postoperative follow-up. A mean follow-up of 48 months was noted in 90.58% (481/531) patients and up to 60 months, it had been reduced to 58.56% (311/531) patients (average 12–180 months).
Discussion
Incidence
In 2012, Duong et al. reported that the incidence of combined malignant and nonmalignant primary spinal tumors differs by age, sex, race, and ethnicity. Their results indicated that malignant primary spinal tumors have been stable throughout the 1999–2007 period. However, the age-adjusted incidence rate for nonmalignant primary spinal tumors (0.76/100,000 persons) was significantly higher than the observed rate for malignant tumors (0.22/100,000 persons). The incidence rate for malignant and nonmalignant primary spinal tumors combined was lowest in children aged 0–9 years (0.23/100,000 persons) and was highest in adults aged 70–79 years (2.53/100,000 persons). The total age-specific incidence rate in children (0–19 years) was 0.27/100,000 persons.[1] The overall incidence rate of nonmalignant spinal tumors, in Croatia, as reported by Materljan et al., is 1.60/100,000 persons.[3] Schellinger et al. found that incidence rates were lowest in children aged 0–19 years and highest in adults aged 75–84 years.[4] Likewise, similar age-specific rate patterns were also found in South Korea and Estonia among patients of the central nervous system tumors.[56] Patil et al., while analyzing a large database source of 19,017 patients of spinal cord tumor resection in the USA, noted that 9% of the patients treated were <18 years of age.[7] Given the above reports, the present study shows a lower incidence (hospital-based) of about 0.24/100,000 persons per year for all spinal tumors and for all ages and for the children (18 years and below), it was lowest 0.014/100,000 persons per year. Again, the malignant spinal cord and bone tumors were incidentally found at 0.08/100,000 person per year whereas benign tumors at 0.17/100,000 persons per year, which is much lower than other reports. Contrary to above reports, the present study reveals the increase (16.38% = 87/531) in the malignant spinal tumors in adults above 60 years [Table 3]. The reasons for the lower incidence could be the study being hospital-based, most-time snow, and mountain-locked for outstate visitor/patients, failure of detection by the investigative tools, migration of the patients to the other centers of the country, and patients shying away from the hospital by resorting to local quack treatment.
Sex
Duong et al. found differences in primary spinal tumors by behavior and sex. Among malignant cases, the frequency was distributed almost equally by sex whereas among nonmalignant cases, approximately 60% were diagnosed in women compared with 40% in men. The male:female ratio for ependymomas was 1.15 and lymphomas 1.76 while for tumors of spinal nerves, it was 1.11 and meningiomas 0.28.[1] A study revealed that 53% were women in all spinal cord tumors.[6] The study of Loblaw, et al. in 2003, observed 57% males among a population of malignant spinal cord compression.[8] Schellinger et al. found that the overall incidence rate among men was 0.70 per 100,000 persons whereas women had an incidence rate of 0.77 per 100,000 persons.[3] The present study observes a sex incidence variation, i.e., men have an incidence of 0.14/100,000 persons per year and women 0.11/100,000 persons per year for all tumors which is much lower than above reports. A male preponderance of 55.93% (297/531) for almost all ages was recorded with a male:female ratio of 1.26:1.00.
Histopathology
The statistical report of Central Brain Tumor Registry United States-2005 reported that the primary tumors of the spinal cord, spinal meninges, and cauda equina (subsequently referred to as “spinal tumors”) are relatively rare.[9] The similar findings were revealed 2010 by the study of Engelhard et al.[10] Tihan et al. reported that intramedullary spinal cord tumors are rare and account for only 5–10% of spinal tumors.[11] Duong et al. reported that among 11,712 primary spinal tumor incident cases, diagnosed from 2004 to 2007, there were 22.0% (2576) malignant cases and 78.0% nonmalignant primary spinal tumors. The most common histologic types of tumors (62%) were myxopapillary ependymomas. The most common spinal tumor was meningiomas (33%) followed by tumors of spinal nerves (27%) and ependymomas (21%). The spinal cord was the origin of 60.5% of all the primary spinal tumors (combined) followed by spinal meninges – 36.0% and cauda equina – 3.5%.[1] Albanese and Platania, 2002, reported that spinal IDEM tumors account for 2/3 of all intraspinal neoplasms and are mainly represented by meningiomas (25–46%) and schwannomas.[12] Researchers in a study of primary spinal cord tumors reported that the spinal cord was the origin of tumor in 70.0% patients, spinal meninges in 26.0%, and cauda equina in 4.0% patients. While the most common histologic type of spinal tumors were meningiomas (29%), this was followed by nerve sheath tumors (24%) and ependymomas (23%).[3] The Croatian study noted that more than 50% of the spinal tumors diagnosed in the population were located in the spinal meninges.[2] However, these results differed from a Norwegian study that found equal proportions of tumors originating from spinal cord and spinal meninges.[13] The present study varied with the above reports, for the spinal cord, was origin to only 19.20% (79 + 23/531 including myxopapillary ependymomas) tumors and observed that 32.58% (173/531) were malignant spinal cord and bone tumors and 67.42% (358/531) were benign [Table 2]. It differed with the above studies in revealing that the most common histologic type of, all 14.87% (79/531) IDIM, cord tumors as IM-ependymoma (7.15% =38/531 excluding myxopapillary variety) followed by the astrocytomas (3.76%=20/531). IDEM tumors occurred in 68.73% (365/531) patients, and the most common tumor type schwannoma was found in 26.74% (142/531) patients followed by the meningiomas 16.57% (88/531) and neurofibromas 12.99% (69/531). The possible explanations for these variations in the present study may be the small sample size and the difference in the size of population.
Management
Frank et al. reviewed a series of 95 patients in which 28% patients were negative for a bone scan, but MRI scan revealed the tumor and the study found a discordance rate of 31% between the two imaging modalities.[14] The study of Patchell et al., in 2005, revealed that MRI with whole spine sagittal T1 and T2 and axial T1 images were superior for detecting metastatic spine as compared to all other investigations.[15] Bilsky et al. observed that though MRI has revolutionized assessment of metastatic spinal tumor, many other imaging modalities play a role in evaluation of metastatic spinal tumor including plain radiographs, bone scan, CT scan, myelogram, and positron emission topography.[16] Algra et al. in a research study reported that bone scans rely on an osteoblastic reaction or bone deposition to detect spinal metastases,[17] while Gosfield et al. stated that patients with rapidly progressive and destructive tumors, such as multiple myeloma, may not be detected by the bone scan.[18] The present study found MRI as the mainstay of the diagnosis in 71.18% (378/531) patients whereas the bone scan (99m Tc-MDP) detected 86.20% (75/87) of all the metastatic and primary malignant spinal bone tumors. The bone scan being so sensitive in this study may be due to the well-established lesions in the neglected patients presenting late to the hospital. It is reported that removal of the tumor was complete in 90% of meningiomas and 94% of schwannomas.[12] Patchell et al. found the direct decompressive surgery plus postoperative radiotherapy superior to treatment with radiotherapy alone for patients with spinal cord compression caused by metastatic cancer.[15] The present study shows that total surgical excision of the tumor was carried out in 81.54% (433/531) patients, in which 1.88% (10/531) were ED-ID, 10.92% (58/531) IDIM, and 68.73% (365/531) were IDEM. The primary malignant vertebral and metastatic deposits were treated with maximum debulking to achieve standard stability by instrumentation (fixation) and optimal results from postoperative chemoradiation therapy.
Primary and secondary malignant compression and risk factors
The findings of Sundaresan and Galicich revealed that the metastatic spinal cord compression was the result of primary cancer from lungs 25%, breast 14%, connective tissue 12%, and prostate 13.04%.[19] McLinton and Hutchison observed that the hematological malignancies such as multiple myeloma and non-Hodgkin's lymphoma accounted for 10% of malignant spine patients in their series,[20] although a study on the possible causes of malignancies of the central nervous system in orchard farmers of Kashmir valley linked the pesticide overuse in brain cancers.[21] Loblaw et al. reported that lung (23%), prostate (18%), and breast cancer (21%) accounted for 61.2% of the malignant spinal cord compression cases.[8] Levack et al. and others observed that more than two-thirds (2/3rd) of the cases of metastatic spinal cord compression occur in the thoracic spine.[228] Walsh et al. reported that 10% of all cancer patients develop spinal metastases during the course of their disease.[23] The SKIMS study showed that overall malignant spinal cord and bone tumors contributed 32.58% (173/531) to all spinal tumors which is higher than most of the above studies. The primary malignant bone tumors were found in 4.14% (22/531) and metastatic deposits occurred in 12.24% (65/531). Among primary malignant tumors of spinal column and soft tissue, the multiple myeloma accounted for 2.25% (12/531) patients. The most common metastatic deposit in the spinal canal was from non-Hodgkin's lymphoma (3.10% = 16/531) as compared to 2.63% (14/531) squamous-celled carcinoma from the lungs and 2.25% (12/531) intraductal carcinoma from breast, mostly in the dorsal and the cervical region [Table 1]. The only case of drop metastasis from the medulloblastoma in a child was located in the cervical spinal level. The overall variation from the other studies, i.e., the most common secondary deposit and primary malignancy being non-Hodgkin's lymphoma and multiple myeloma, may be due to increase in the blood-borne malignancies in this region. The possible cause could be high pesticide (chemicals) use in orchards which needs to be assessed.
Surgical outcome
Researchers reported the in-hospital mortality rate of 0.55% and a complication rate of 17.5% in spinal cord tumors such as ependymoma, astrocytoma, and hemangioblastomas.[7] A study reported that meningiomas had a morbidity rate of 5% and a mortality rate of 0% while for schwannomas, surprisingly it was 0% morbidity and 6% mortality. Almost all the patients experienced a significant neurological improvement after surgery, with a percentage of Nurick Grades 1 and 2 of 68% among patients with meningiomas and 66% patients with schwannomas.[12] Patchell et al. studied 101 metastatic spinal cord compressions in which 84% (42/50) had postoperative good recovery (able to walk) compared to only 57% (29/51) postradiotherapy good recovery. The 30-day mortality rates were only 6% in the surgery group whereas a higher mortality of 14% was seen in the radiation group.[15] Gokaslan et al. also reported postsurgical functional and neurologic improvements in 50–76% of spinal metastatic patients with a postsurgical complication rate of 30%.[24] The study of metastatic spinal tumors by Bilsky et al. depicted 58% recurrence after 6 months, 69% at 1 year, and 96% after 4 years. There was a mortality of 13% which was attributed to the medical or oncologic condition of the patients.[16] Sundaresan et al. have noted significant local recurrence rates in malignant spinal resections causing cord compressions.[25] The present study revealed no mortality in IDEM and IDIM tumors, but a mortality of only 3.20% (17/531) was recorded in metastatic spines (i.e., 26.15% = 17/65) and 16.57% (88/531) patients developed various complications. About 76.08% (404/531) patients showed postoperative improvement, 27.68% (147/531) of these showed Nurick Grade 0 (good recovery) and 4.14% (22/531) worsened postoperatively. The postsurgical recurrences and residual tumors were noted in 4.90% (26/531) patients within 9 months of postoperative follow-up. Zadnik et al. reviewed the genetic basis of treatment of spinal cord tumors and concluded that adjuvant radiation and chemotherapy are the treatment of choice after surgical resection of spinal cord astrocytomas and ependymomas whereas the targeted therapeutics is under investigation.[26] The present study at SKIMS subjected about 16.38% (87/531) patients to the adjuvant therapy in the form of radiotherapy and chemotherapy within 1–3 months of postoperative follow-up. Most of these patients were primary malignant vertebral and metastatic tumors. Besides all astrocytomas and myxopapillary ependymomas were also subjected to the adjuvant therapies. Avila et al. reviewed and analyzed practice patterns for stabilization and fusion after ID spinal tumor resection in 1288 adults where 8.1% (104 cases) required fusion. The median follow-up of all the series was 24 months (range 1.5–180). The criteria for fusion that were common in most cases were previous deformity (i.e., kyphosis in the cervical spine), 3 or more levels of laminectomy, laminectomy encompassing a spinal junction, “young adults” (33 ± 4.2 years), facetectomy ≥50% (unilateral or bilateral), persistence of deformity after 1 year of the surgery, and C2 laminectomy.[27] The SKIMS study revealed that posterior fusion with Harrington rods and transpedicular screw-fixation in the cervical and dorsal spine (including lumbar 1 and 2) was performed only if 3 or more than 3 laminae were removed, unilateral or bilateral lateral ½ facetectomy was done and laminectomy at cervicodorsal or dorsolumbar regions were performed to excise a tumor. All cervical, dorsal, and dorsolumbar vertebral primary tumors, metastatic deposits, extensive IDEM tumors with vertebral invasion, and vascular tumors with collapse of vertebral bodies were approached by the anterior, lateral, anterolateral, and posterolateral approaches to perform a decompressive corpectomy. The anterior cervical and lateral clavico-sternotomy approaches were used to do a cervical corpectomy with a cage and plate fixation. Traul et al. in a review discussed the ID spinal cord tumors in terms of epidemiological, radiographic, and histological characteristics. Surgical and adjuvant treatment strategies are also reviewed.[28] Zong et al. reported treatment results of 122 IDEM cases with minimally invasive surgery (MIS) and non-MIS. The fusion results showed that laminectomy + pedicle screw fixation showed better postoperative spinal stability than hemilaminectomy and laminectomy in two or more spinal operative segments. In the evaluation of long-term clinical efficacy at the time of final follow-up (12–60 months, average 28 months), improvement was regarded as effectivity whereas no change and deterioration were regarded as nullity. It is generally known that pedicle screw fixation offers a significant biomechanical advantage and is superior to other techniques regarding the promotion of mechanical strength. As a result, great internal stability is observed.[29] The present study at SKIMS revealed that hemilaminectomies were performed in 11.50% (42/365) cases of IDEM. While lateral position was used for the modified posterolateral rib resection/intercostal transthoracic intrapleural, modified posterolateral rib resection/intercostal transthoracic extrapleural retroperitoneal, anterolateral retroperitoneal and transaxillary (modified SKIMS) approaches for decompressive corpectomy and instrumentation with dynamic compression plating and rod and cage fixation for interbody fusion in the dorsal and lumbar regions. The materials for the fusion used were either autologous (iliac crest graft, fibular bone graft, rib graft, spinous process graft, etc.) or synthetic materials. A mean follow-up of 48 months was noted in 90.58% (481/531) patients and up to 60 months, it fell to 58.56% (311/531) patients (average 12–180 months).
Conclusion
The SKIMS study provided an insight into incidences of primary benign, malignant and metastatic spinal cord, meningeal, and vertebral column tumors on the basis of histopathological evidence. The regional incidence of these tumors is comparably lower than other comparable studies. It showed that secondary or metastatic spinal tumors, which are on rise, occur in elderly and male population. The surgical outcome in terms of tumor excision, functional recovery, and spinal stability was better in IDEM group than in IM, ED-ID group, primary and metastatic vertebral column tumor groups. The treatment of choice for the malignant and metastatic tumor group of spinal cord and column was the maximum resection with adjuvant chemoradiation therapy. A mean follow-up of 48 months (range 12–180 months) was recorded. This study, in the absence of a community-based data, may serve as a hospital-based regional epidemiological study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors are thankful to Moji, Sameena, Huwa, Aisha, SaimUl, RohUl, and Adam for their help in successfully bringing out this manuscript.
References
- Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer. 2012;118:4220-7.
- [Google Scholar]
- The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100.
- [Google Scholar]
- Epidemiology of central nervous system tumors in Labin area, Croatia, 1974-2001. Croat Med J. 2004;45:206-12.
- [Google Scholar]
- Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173-9.
- [Google Scholar]
- Central nervous system tumors among Koreans – A statistical study on 697 cases. J Korean Med Sci. 1989;4:77-90.
- [Google Scholar]
- Epidemiology of primary central nervous system tumors in Estonia. Neuroepidemiology. 2000;19:300-11.
- [Google Scholar]
- Complications and outcomes after spinal cord tumor resection in the United States from 1993 to 2002. Spinal Cord. 2008;46:375-9.
- [Google Scholar]
- A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15:211-7.
- [Google Scholar]
- Central Brain Tumor Registry of the United States. In: CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors in the United States, 1998-2002. Hinsdale, IL: CBTRUS; 2005.
- [Google Scholar]
- Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine. 2010;13:67-77.
- [Google Scholar]
- Pathologic and epidemiologic findings of intramedullary spinal cord tumors. Neurosurg Clin N Am. 2006;17:7-11.
- [Google Scholar]
- Spinal intradural extramedullary tumors. Personal experience. J Neurosurg Sci. 2002;46:18-24.
- [Google Scholar]
- Trends in incidence of brain and central nervous system tumors in Norway, 1970-1999. Neuroepidemiology. 2004;23:101-9.
- [Google Scholar]
- Detection of malignant bone tumors: MR imaging vs scintigraphy. AJR Am J Roentgenol. 1990;155:1043-8.
- [Google Scholar]
- Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643-8.
- [Google Scholar]
- Do metastases in vertebrae begin in the body or the pedicles. Imaging study in 45 patients? AJR Am J Roentgenol. 1992;158:1275-9.
- [Google Scholar]
- Comparison of radionuclide bone scans and magnetic resonance imaging in detecting spinal metastases. J Nucl Med. 1993;34:2191-8.
- [Google Scholar]
- Treatment of spinal metastases by vertebral body resection. Cancer Invest. 1984;2:383-97.
- [Google Scholar]
- Malignant spinal cord compression: A retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94:486-91.
- [Google Scholar]
- Pesticides and brain cancer linked in orchard farmers of Kashmir. Indian J Med Paediatr Oncol. 2010;31:110-20.
- [Google Scholar]
- Don't wait for a sensory level – Listen to the symptoms: A prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14:472-80.
- [Google Scholar]
- Anterior approaches to the thoracic spine in patients with cancer: Indications and results. Ann Thorac Surg. 1997;64:1611-8.
- [Google Scholar]
- Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599-609.
- [Google Scholar]
- Treatment of neoplastic spinal cord compression: Results of a prospective study. Neurosurgery. 1991;29:645-50.
- [Google Scholar]
- Spinal cord tumours: Advances in genetics and their implications for treatment. Nat Rev Neurol. 2013;9:257-66.
- [Google Scholar]
- Fusion after intradural spine tumor resection in adults: A review of evidence and practices. Clin Neurol Neurosurg. 2015;138:169-73.
- [Google Scholar]
- Treatment results in the different surgery of intradural extramedullary tumor of 122 cases. PLoS One. 2014;9:e111495.
- [Google Scholar]






