Translate this page into:
Bilateral Facial Palsy a rare presenting symptom of acute lymphoblastic leukemia with CNS and BM Relapses
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Acute lymphoblastic leukemia (ALL) is a common hematological malignancy of childhood. Neurological deficits are common in leukemia. These complications commonly occur due to central nervous system (CNS) spread of the disease or rarely by the toxic effect of chemotherapeutic drugs. Facial palsy may be a presenting symptom of disease or relapse of disease and in the majority of times, it is unilateral. Here, we report a case of pre-B cell who presented with bilateral lower motor neuron facial nerve paralysis because of meningeal spread of CNS relapse in a patient who had bone marrow (BM) and CNS relapse while on maintenance therapy.
A 15-year-old boy was diagnosed as pre-B cell ALL in July 2012. At the time of presentation, extra-medullary spread of disease was not present, computerized tomography (CT) scan of the head was normal and cerebrospinal fluid (CSF) analysis did not show CNS involvement of the disease. He obtained complete hematological remission. The patient had received standard induction regimen constituting of daunorubicin, L-asparaginase, vincristine, and prednisolone followed by cyclophosphamide and arabinoside-C. Interim maintenance was done using 6-mercaptopurine, methotrexate, vincristine, and prednisolone. This was followed by re-induction using the induction regimen and finally maintenance with 6-mercaptopurine, methotrexate, vincristine, and prednisolone. CNS prophylaxis was done using a triple intrathecal regimen of hydrocortisone, methotrexate and arabinoside-C for the first 6 doses at weekly interval followed by intrathecal methotrexate monthly for 3 months and then every three months. The last dose of vincristine was given in January 2013, and he was not suffering from symptoms related to vincristine toxicity such as constipation, jaw pain, and abdominal distension. In June 2013 while on maintenance therapy he was admitted with complaints of generalized headache, vomiting, deviation of angle of mouth toward left side on attempted while speaking, and inability to close his right eye (House and Brackmann Grade VI). Over the next 5 days, his left side of the face was also involved (House and Brackmann Grade VI). [Figure 1]. Now he was unable to close both his eyes properly and was not able to blow or whistle. There was no history of hyperacusis and loss of taste sensation on the tongue. Lacrimation was normal. There was no history suggestive of ataxia or seizures. He denied any history of trauma, insect bite, or rashes. On examination, his vital parameters were stable. The cardiac and chest auscultation were normal. His higher mental functions were normal. He had lost frowning over the forehead on an attempt to look upward. He was unable to close his both eyes properly, and Bells phenomenon was positive. Bilateral papilledema was present. Other cranial nerves examinations were normal. Sensory and motor system examinations were within normal limit. Plantar reflex was flexor bilaterally. He did not show any sign of meningeal irritation. The examination of head, eyes, ears, nose, and throat was normal. There was no thyromegaly, parotid gland enlargement, lymphadenopathy, or mucosal rugosities. Liver and spleen were not palpable.
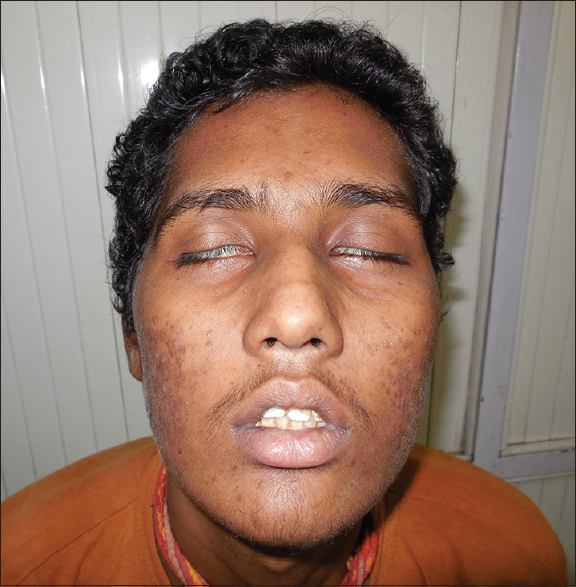
- Bilateral lower motor neuron type of facial palsy (House and Brackmann Grade VI)
Investigations revealed hemoglobin of 7.6 g/dL, platelets 51,000/mm3, and white blood cells (WBCs) were 2010/mm3 with 69.2% of lymphocyte and 28% neutrophils. The peripheral blood film showed pancytopenia and few blast cells. BM aspiration showed hypercellular marrow with 84% of blast cells, indicating relapse of ALL. His renal and liver functions were within the normal limit. CSF analysis showed protein 233 mg/dL, sugar 23 mg/dL, WBCs 2700/mm3 and red blood cells 20/mm3. Giemsa stain showed lymphocyte and atypical cells with high N/C ratio and clumped coarse chromatin suggestive of malignancy. CSF culture was sterile. TB-PCR was negative. VDRL test, HIV antibody test, and oligoclonal bands were also negative. CT scan of the head was normal. Magnetic resonance imaging with gadolinium showed enhancement of meninges. In a case of ALL, these findings indicated CNS meningeal spread of ALL relapse. Sequential administration of methotrexate and L-asparaginase was undertaken with CNS radiation. He did not show any improvement and expired after 2 months.
Bilateral simultaneous facial palsy is a rare entity and has an incidence of only 1 per 5 million populations per year.[1] It is rarely idiopathic (under 20%), whereas unilateral is mostly idiopathic (over 50%).[2] It may be the presenting feature of a potentially life-threatening illness. The differential diagnoses of bilateral facial palsy include congenital, traumatic, infectious, neurological, metabolic, neoplastic, and toxic causes. Infective causes include postinfluenza, infectious mononucleosis, HIV infection, Lyme disease, Bannwarth's syndrome, Guillain-Barre syndrome, syphilis, brainstem encephalitis, HTLV-1 infection, and poliomyelitis.[3] Other rare causes are diabetes, acute porphyria, sarcoidosis, amyloidosis, Miller Fisher syndrome, Hansen disease, tubercular meningitis, and lupus.[1]
Among the tumors, parotid tumors, the vestibular schwannoma, glomus tumors are common to involve facial nerve unilaterally. These tumors directly injure the nerve. In the case of leukemia, the systemic manifestations may lead to facial paralysis. Most of the patients with leukemia and facial palsy have leukemic cells in the CSF or disease activity in other organs.[4]
ALL metastasis to CNS can lead to various neurological symptoms. These symptoms are seizures, meningitis, focal neurological deficits, isolated or multiple cranial nerve palsy, posterior reversible encephalopathy syndrome, cerebrovascular disease, neurocognitive impairments, methotrexate toxicity: Leukoencephalopathy (acute confusion, seizures, and encephalopathy) and secondary CNS malignancies: Meningiomas and gliomas: Cranial tumors occurs following high-dose CNS radiation.[5] Patients can present with these symptoms at the time of first presentation or in a relapse of the disease. CNS is the most common site of extramedullary relapse of disease in ALL. CNS relapses can occur isolated or in combination with BM relapse. Patients with hyperleukocytosis, T-cell ALL, philadelphia chromosome, MLL gene rearrangement t(4;11), and the presence of leukemic cells in the CSF have been noted to have a greater risk of CNS relapse.[5] Traumatic lumbar puncture can introduce peripheral blasts into the CSF, and there is more chance of meningeal infiltration by blast cells.[5] Children and preschool factors are also at higher risk as compared with adolescents and young adults, probably because of the greater relationship between blood flow and brain tissue.[4]
Facial palsy in lymphoid malignancies has been reported with accompanying meningeal involvement.[6] Acute otomastoiditis subsequent to leukemic infiltration of the temporal bone may be implicated with facial and acoustic nerve paralysis. The presence of neoplastic lymphocytes or myelocytes in arachnoid tissue cause meningeal leukemia. These malignant cells proliferate at shallow walls of veins and extending through the surface to the arachnoid emerging arteries, veins, arterioles, venules, and that cross the brain. Meningitis in leukemia occurs secondary to cerebral hypoperfusion because leukemic cells reduce the caliber of these vessels. Neuropathy occurs due to compression and damage of the nerve and their vessel by infiltration of leukemic cells,[7] which might have been a mechanism in our case. Our patient was a diagnosed case of ALL and obtained complete remission, presented with bilateral facial palsy, having high level of protein and malignant cells in CSF, with magnetic resonance imaging showing enhancement of meninges suggestive of meningeal spread of ALL relapse.
To conclude, the simultaneous bilateral facial palsy is very rare presenting symptom, some underlying severe disease and rarely idiopathic. Leukemic relapse should be considered as differential diagnoses of bilateral facial palsy. CNS chemoprophylaxis, cranial irradiation, and avoidance of bloody tap of lumber puncture can reduce the chances of relapse.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bilateral facial paralysis: A case presentation and literature review. J Otolaryngol. 1992;21:44-7.
- [Google Scholar]
- Bilateral simultaneous facial paralysis – Differential diagnosis and treatment options. A case report and review of literature. Acta Otorhinolaryngol Belg. 2003;57:139-46.
- [Google Scholar]
- Bilateral peripheric facial nerve palsy in acute linfoid leukemia: A case report. Rev Bras Otorrinolaringol. 2004;70:261-64.
- [Google Scholar]
- Acute lymphoblastic leukemia: What have we learned about the effects of this disease and its treatment on the nervous system. In: Faderl S, ed. Novel Aspects in Acute Lymphoblastic Leukemic. Croatia: InTech Open; 2011. p. :73-98.
- [Google Scholar]
- Cranial nerve involvement in children with leukemia and lymphoma. J Clin Oncol. 1983;1:542-5.
- [Google Scholar]
- Adult T-cell leukemia initially manifesting as facial diplegia. Am J Hematol. 1989;32:61-5.
- [Google Scholar]





