Translate this page into:
Optic nerve sonography: A noninvasive means of detecting raised intracranial pressure in a resource-limited setting
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The aim was to assess the use of optic nerve sonography (ONS) as a quick, noninvasive diagnostic test tool for detecting raised the intracranial pressure (ICP).
Materials and Methods:
A prospective blinded observational study was conducted at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Nigeria. The study population consisted of 160 adult patients referred to the radiology department for cranial computed tomography (CT) scan. There were 80 subjects and 80 controls. Optic nerve sheath diameter (ONSD) was measured by a radiologist using a 7.5 Megahertz ultrasound probe while cranial CT was reviewed by other radiologists blinded to the ONSD.
Results:
Sixty-nine subjects (86.3%) had intracranial space occupying lesions (SOL) with cranial CT confirmed features of increased ICP, mean binocular ONSD of 5.7 ± 0.59 mm while 11 (13.7%) had intracranial SOL without any cranial CT evidence of increased ICP, mean binocular ONSD of 4.8 ± 0.39 mm. The difference of mean ONSD of the two groups was statistically significant (P = 0.0001). The controls had a mean binocular ONSD of 4.5 ± 0.22 mm and the difference in mean binocular ONSD for subjects with raised ICP and the controls were also statistically significant (P = 0.0001). A cut-off value of 5.2 mm (sensitivity 81.2% [95% confidence interval (CI): 69.9–89.6], specificity 100% [95% CI: 71.5–100]) was obtained from the receiver operator characteristics curve as the mean binocular ONSD that best predicts raised ICP confirmed by at least a sign on cranial CT.
Conclusions:
Optic nerve sonography can differentiate between normal and elevated ICP and may serve as a useful screening tool in resource-limited practice.
Keywords
Optic nerve sheath diameter
raised intracranial pressure
resource-limited settings
ultrasound
Introduction
Raised intracranial pressure (ICP) requires early detection in order to institute prompt medical or surgical intervention. Hence, a quick, noninvasive and fairly reliable means of determining raised ICP is highly beneficial in this critical situation, especially in resource-limited settings. Recent studies have shown that due to the anatomical relationship of the optic nerve complex to the subarachnoid space within the brain, an increase in ICP results in increased distention of the optic nerve sheath and hence an increase in optic nerve sheath diameter (ONSD).[123456] The proximal portion of the optic nerve complex has been found to be most sensitive to changes in ICP and studies have taken ONSD measurements at a retrobulbar position 3 mm behind the globe.[134] This portion demonstrates the optimum sonographic contrast of the hypoechoic optic nerve complex within the echogenic retrobulbar fat and provides a noninvasive means of detecting raised ICP. Some studies in other parts of the world have also observed that alterations in ONSD strongly correlated with neuroimaging findings on cranial computed tomography (CT) in patients with elevated ICP.[4789] The purpose of this study was to assess the use of optic nerve sonography (ONS) for predicting raised ICP in a resource-limited environment.
Materials and Methods
This prospective blinded observational study was carried out between May 2011 and April 2012 at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Nigeria. Approval of the study protocol was granted by the hospital ethics and research committee. The study population included 160 patients aged 18 years and above who were referred to the Radiology Department for Cranial CT scan. Eighty of the patients had a clinical suspicion of an intracranial space occupying lesion (SOL) with possibly raised ICP and served as subjects. To minimize observer bias, the subjects had the ONSD measured before the cranial CT scan was done. Eighty patients who were also referred to the Radiology department for cranial CT scans for other indications without a clinical suspicion of raised ICP served as controls and had their ONSD measured after the cranial CT examination showed no intracranial SOL or raised ICP features. The ONSD was measured by a radiologist and the radiological findings on the cranial CT images were reported by other radiologists who were blinded to the ONSD measurements.
Optic nerve sonography was performed using a 7.5 MHz linear probe (Ultrasound unit: Mindray real-time ultrasound scanner model DC-6; Shenzhen, China). Patients were placed in supine position, and a thick layer of sterile coupling gel was applied to the closed, upper eyelid. The probe was placed on the temporal area of the eyelid, and an axial view of the orbit was obtained displaying the entry of the optic nerve into the globe. The image was frozen, and the cursors were placed on the outer contours of the dural sheath, at a retrobulbar position 3 mm behind the globe and perpendicularly to the optic nerve axis. ONSD was calculated as the horizontal distance between the 2 cursors [Figure 1]. The ONSD for each eye was measured 3 consecutive times, and the mean ONSD was taken in order to minimize intra-observer error. A mean binocular ONSD was calculated from the mean ONSD of each eye. Thereafter the cranial CT scan was done, and the findings were used as a reference standard to assess raised ICP. Cranial CT findings that suggested raised ICP includes effacement of sulci, midline shift, dilated ventricles, the collapse of ventricles, or compression of cisterns.
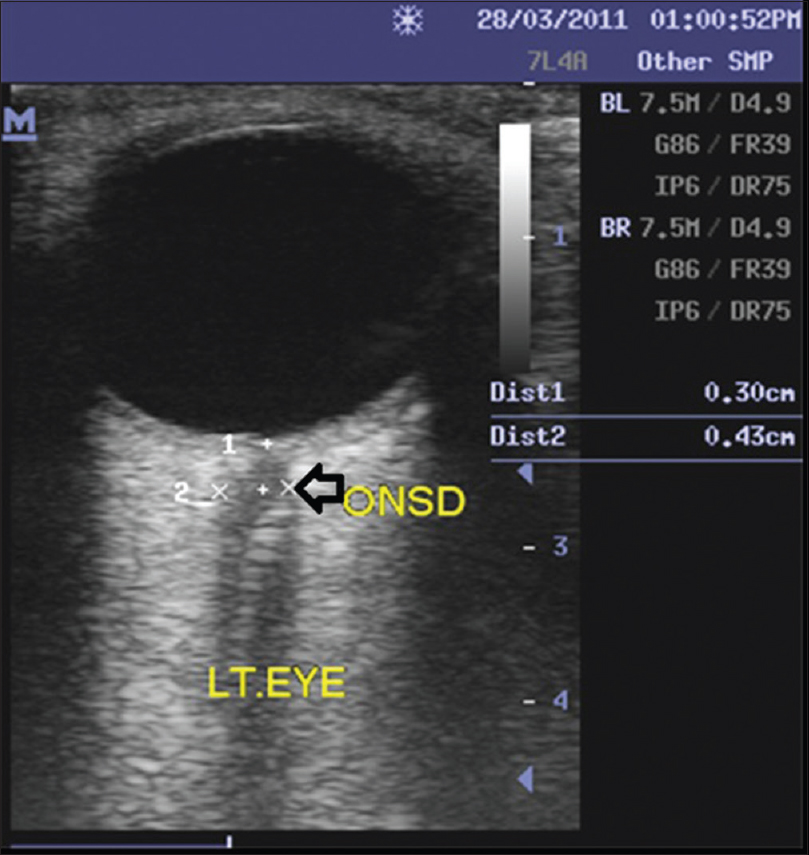
- Axial ultrasound scan image of the left eye showing the hypoechoic optic nerve complex within the echogenic retrobulbar fat. Optic nerve sheath diameter measured at a point 3 mm behind the globe was 4.3 mm
The data were entered into a spreadsheet and analyzed using Statistical Package for Social Sciences (SPSS) for Windows, Version 16.0 (Chicago, SPSS Inc.). Descriptive and inferential statistical methods were carried out, P values and confidence intervals (CIs) were determined to assess the level of significance. P <0.05 was considered to be statistically significant. A receiver operator characteristic (ROC) curve was obtained to determine the cut-off value for the optimum diagnostic accuracy of ONS in predicting raised ICP as evidenced by at least a sign on cranial CT.
Results
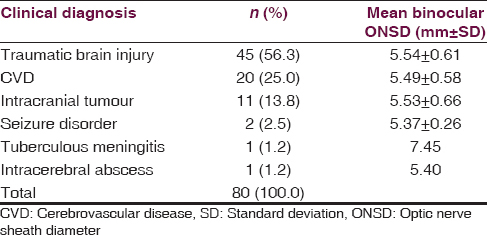
Of the 80 subjects, 45 (56.3%) had a clinical diagnosis of posttraumatic intracranial SOL due to head injury while 35 (43.7%) had suspected nontraumatic intracranial SOL. The various clinical diagnoses of the subjects at presentation and the corresponding mean ONSD are shown in Table 1. The difference in mean ONSD between traumatic and nontraumatic lesions was not statistically significant (P = 0.7578).
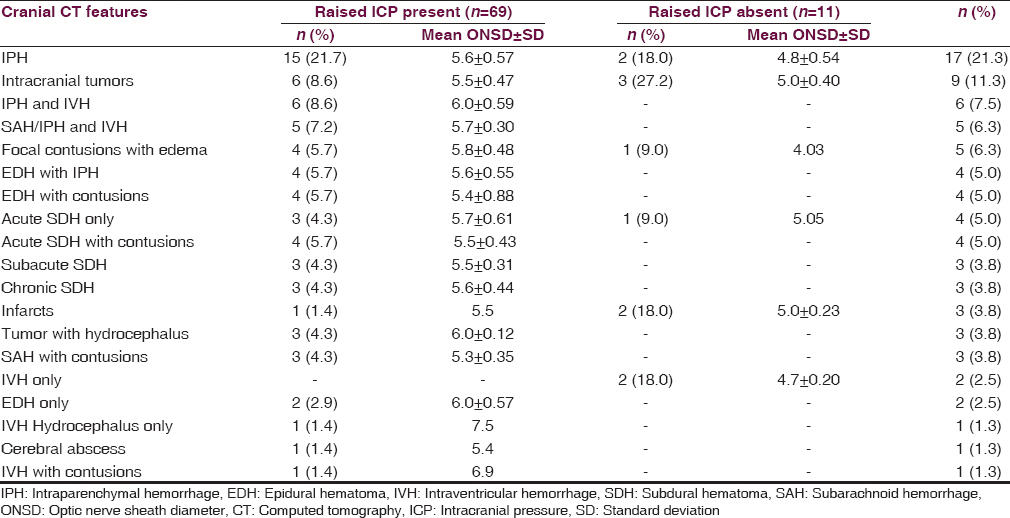
The cranial CT scan features of increased ICP amongst the subjects with intracranial SOL included sulci effacement (n = 65, 81.3%), ≥3 mm midline shift (n = 35, 43.8%), dilated ventricles (n = 37, 46.3%) compression of the ventricles (n = 41, 51.3%) and compression of basal cisterns (n = 28, 35.0%). Among the 80 subjects, 69 (86.3%) had intracranial SOL with cranial CT confirmed features of increased ICP and a mean binocular ONSD of 5.7 ± 0.59 mm while 11 (13.7%) patients had intracranial SOL without any cranial CT evidence of increased ICP and a mean binocular ONSD of 4.8 ± 0.39 mm. The controls had a mean binocular ONSD of 4.5 ± 0.22 mm. The CT diagnoses and corresponding mean binocular ONSD measurements in the subjects are shown in Table 2. The difference in mean binocular ONSD for subjects with raised ICP and the controls was statistically significant (P = 0.0001).
A box plot [Figure 2] shows the relationship between the mean binocular ONSD of the subjects and the presence or absence of cranial CT features of raised ICP. Comparison of mean ONSD of subjects with and without cranial CT signs of raised ICP was statistically significant (P = 0.0001).
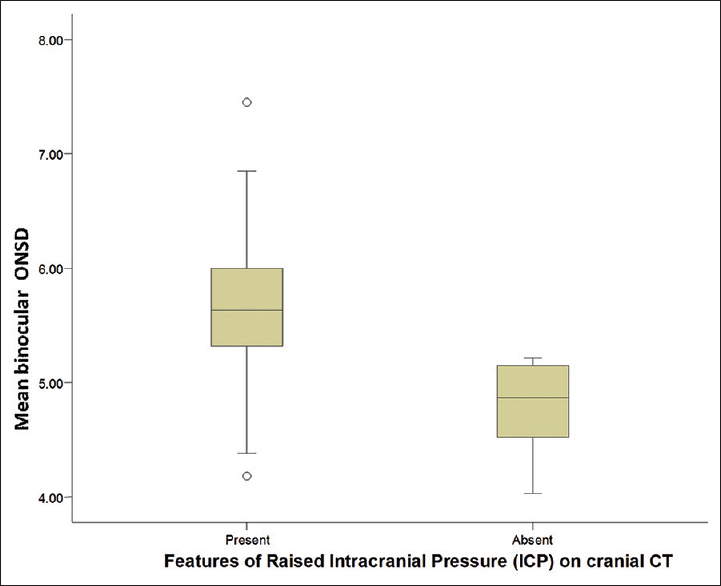
- Box plot shows the relationship between mean binocular optic nerve sheath diameter and presence or absence of raised intracranial pressure features on cranial computed tomography among subjects
The ROC curve [Figure 3] was obtained to determine the cut-off value for the optimum diagnostic accuracy of ONS in predicting increased ICP. The cut-off value that best predicts raised ICP as evidenced by at least a sign on cranial CT was a mean binocular ONSD of 5.2 mm (sensitivity of 81.2% [95% CI: 69.9–89.6] and specificity of 100% [95% CI: 71.5–100]). The area under the ROC curve obtained was 0.90 (95% CI: 0.84–0.97).
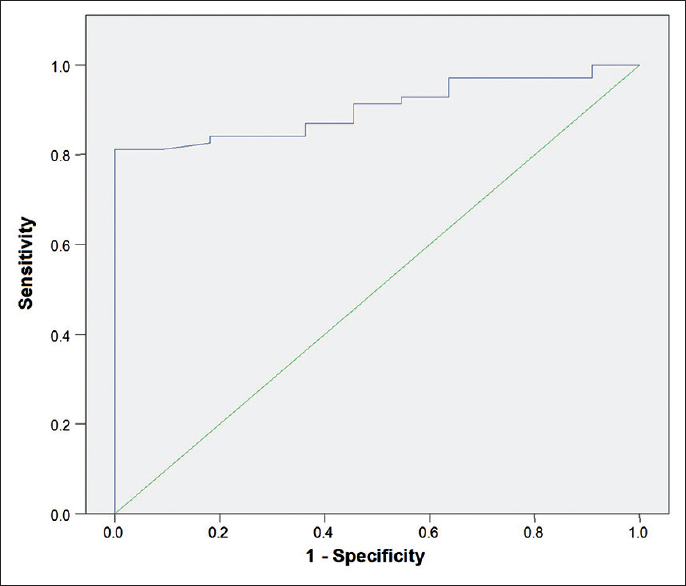
- Receiver operator characteristics curve the cut off value that maximizes sensitivity and specificity corresponds to 5.2 mm (sensitivity of 81.2% [95% confidence interval (CI): 69.9–89.6] and specificity of 100% [95% CI: 71.5–100]) area under curve = 0.90 (95% CI: 0.84–0.97)
Discussion
The diagnosis of raised ICP in low and middle-income countries often present with significant challenges due to limited resources. The use of intraventricular or subdural catheters is the current gold standard for diagnosing raised ICP. However, this technique is invasive and exposes patients to intracerebral complications such as infection and hemorrhage[1011] may not be feasible in patients with bleeding tendencies[1213] and also requires neurosurgical expertise. Noninvasive imaging modalities that can be used include cranial CT scan, magnetic resonance imaging (MRI) and transcranial Doppler ultrasonography. These are expensive equipments, which are not readily available in resource-limited settings. Ophthalmoscopy shows increased ICP in late stages when papilledema has already developed.[1114] Early diagnosis of raised ICP is necessary for optimal patient management and survival. Hence, the need for readily available, cost effective, fast, noninvasive means of initial assessment cannot be over emphasized. Ultrasonography has become an essential component of emergency service[15] and most medical facilities have ultrasound scanning machines. ONS is an emerging area for its clinical application in low and middle-income settings.
In this study, there was a remarkable difference in the mean binocular ONSD of patients with confirmed cranial CT scan features of increased ICP and the control group with no such findings. This was statistically significant (P < 0.001) and agrees with findings in other studies.[478] Previous studies[1471617] have suggested that ONSD values >5 mm should prompt suspicion of elevated ICP. In this study, the cut-off value that maximized sensitivity and specificity of ONSD in detecting raised ICP confirmed by at least a sign of increased ICP on cranial CT scan was a mean binocular ONSD of 5.2 mm which is in agreement with the Moretti et al.[5] study. It was higher than a cutoff value of ≥4.8 mm quoted by Rajajee et al.[18] and ≥5 mm quoted by Major et al.,[1] Kimberly et al.,[16] and Blaivas et al.[17] However, it was lower than 5.7 mm indicated by Tayal et al.[19] and Geeraerts et al.[2021] The varying cut-off values may also be due to the data obtained in different environments for varying study population sizes.
Raised ICP can arise from intracranial SOL of traumatic and nontraumatic origin. The most common clinical diagnosis that was associated with a suspicion of raised ICP in this study was a traumatic brain injury. This often results from road traffic injuries and patients often present at the emergency center as mass casualties. The physician or surgeon has to promptly identify those patients who require treatment most urgently. In such critical situations, ONS may play a beneficial role as a screening tool for initial assessment of raised ICP and facilitate triage since the technique does not require elaborate patient preparation and can be done as a bedside procedure. This provides a high index of suspicion of raised ICP when combined with clinical signs and symptoms.
The most common non-traumatic clinical diagnosis in this study was spontaneous intracranial bleeds from cerebrovascular disease and the highest ONSD value was recorded in a patient with hydrocephalus following tuberculous meningitis. Interestingly it was observed that whenever raised ICP sets in, the ONSD was elevated, irrespective of the underlying pathology. Moreover, comparison of the mean ONSD between traumatic and nontraumatic clinical indications was not statistically significant (P = 0.7578). This buttresses the fact that the practical application of ONS is not limited to trauma cases but can also benefit patients at risk of developing raised ICP from nontraumatic causes such as spontaneous intracranial bleeds, neoplastic and infective brain lesions.
Optic nerve sonography is a fast, noninvasive, cost effective, easily reproducible imaging modality. It does not utilize ionizing radiation or contrast medium, thereby eliminating the deleterious effects of radiation and risk of contrast reaction. However, ONS is not meant to replace other sophisticated radiodiagnostic equipment such as the CT scan and MRI, which give information about the etiology of raised ICP and the presence and extent of an intracranial SOL. It is also important to note that ultrasound examinations are operator dependent, and this may be regarded as a limitation.
Conclusion
Optic nerve sonography can identify a difference between normal and elevated ICP and may be a useful screening tool in resource-limited practice. Further studies correlating ONSD measurements with intraventricular or subdural catheter diagnosis of raised ICP should be done in our environment.
Acknowledgment
We acknowledge the receipt of part funding by the Management of the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Ultrasound measurement of optic nerve sheath diameter in patients with a clinical suspicion of raised intracranial pressure. Emerg Med J. 2011;28:679-81.
- [Google Scholar]
- Effect of intracranial pressure on the diameter of the optic nerve sheath. J Neurosurg. 2008;109:255-8.
- [Google Scholar]
- The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18:323-8.
- [Google Scholar]
- The role of optic nerve ultrasonography in the diagnosis of elevated intracranial pressure. Emerg Med J. 2007;24:251-4.
- [Google Scholar]
- Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care. 2009;11:406-10.
- [Google Scholar]
- Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009;26:630-4.
- [Google Scholar]
- Transorbital sonographic monitoring of optic nerve diameter in patients with severe brain injury. Transplant Proc. 2006;38:3700-6.
- [Google Scholar]
- Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. 2008;12:R67.
- [Google Scholar]
- Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813-21.
- [Google Scholar]
- Sonographic evaluation of optic nerve diameter in children with raised intracranial pressure. J Ultrasound Med. 2005;24:143-7.
- [Google Scholar]
- Perioperative head injury management in the multiply injured trauma patient. Int Anesthesiol Clin. 2002;40:31-52.
- [Google Scholar]
- Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patient study. Pediatr Radiol. 1996;26:706-10.
- [Google Scholar]
- Model curriculum for physician training in emergency ultrasonography. Ann Emerg Med. 1994;23:95-102.
- [Google Scholar]
- Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201-4.
- [Google Scholar]
- Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003;10:376-81.
- [Google Scholar]
- Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506-15.
- [Google Scholar]
- Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508-14.
- [Google Scholar]
- Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. 2007;33:1704-11.
- [Google Scholar]
- Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008;34:2062-7.
- [Google Scholar]






