Translate this page into:
To study the correlation of serum S-100 protein level with the severity of stroke and its prognostic implication
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
This study investigated correlation between mortality, stroke subtype and stroke severity with serum S-100 protein level prior to the treatment of the patients admitted to the emergency department and diagnosed with a stroke.
Methods:
Pretreatment sample were collected from the patients (n = 142) to determine S-100 protein level, age and sex-matched healthy individuals (n = 40) served as control. All patients had cranial computerized tomography scan/magnetic resonance imaging in the first 24 h. The neurological evaluation was made with the National Institute of Health Stroke Scale (NIHSS) in the acute stage.
Results:
Compared with controls, S-100 protein level were significantly higher in the stroke groups. In stroke groups, S-100 protein level was more significantly higher in the ischemic group than hemorrhage and transient ischemic attack group and highest in expired patients.
Conclusion:
Serum S-100 protein measurement can be used as an early marker of brain damage. There is a role of S-100 protein as a co-predictor of outcome in patients with acute stroke.
Keywords
National Institute of Health Stroke Scale
prognosis
S-100 protein
stroke
Introduction
Stroke is a devastating condition encompassing a wide range of pathophysiological entities that include thrombus, hemorrhage, and embolism. Currently, diagnosis of stroke relies on physical examination and is further supplemented with various neuro imaging technique. A single set or multiple set of blood biomarkers that could be used differentiate between stroke subtypes or even predict an initial/reoccurring stroke could be extremely valuable.
In recent year, neurobiochemical markers have gained particular attention in the determination of brain damage from stroke. Stroke is a third leading cause of morbidity and mortality in the world. Hence, there is a need of determination of these markers make it possible to reach a faster diagnosis and to start treatment earlier. Elevated S-100 protein level in cerebrospinal fluid (CSF) in acute cerebral damage have been reported earlier in stroke,[123] but the difficulty in collecting CSF sample make researchers to examine the biochemical markers in the blood.
S-100 protein is a low molecular weight protein (approximately 10 kda) that belong to a multigenic family of calcium-mediated protein, so named their solubility in 100% ammonium sulfate.[4] S-100 protein is released into CSF upon structural damage to the neuronal cells, but the underlying mechanism of passage through the blood-brain barrier has not seen clearly elucidated. The correlation of S-100 is 40 fold higher in CSF then serum. The biomarkers is not affected by hemolysis and has exceptional stability[5] giving it appeal for use as clinical biomarkers.
Several studies have demonstrated the serum S-100 protein concentration are increased significantly following stroke.[678910111213] S-100 protein level measure before specific treatment was given and mortality stroke subtype and severity were examined in patients with stroke admitted to hospital emergency department.
Patients, Methods, and S-100 Protein Measurement
This was a prospective observational study with a definite diagnosis of stroke made both clinically and radiologically, conducted among patients admitted in Mahatma Gandhi Hospital (MGH) Jodhpur.
This study was approved by the Local Research Ethics Committee. Inclusion criteria for the study were patients of acute stroke admitted within 48 h of onset, diagnosis confirmed by computerized tomography/magnetic resonance imaging and patients above the age of 18 years. Exclusion criteria were patients with peripheral vascular disease, systemic inflammatory disorder or tumor, patients whose National Institute of Health Stroke Scale (NIHSS) score could not be calculated at the time of admission. Venous samples were drawn within 48 h of the onset of symptoms and sent for routine blood examinations including measurement of the protein level. The concentration of protein in blood samples was measured in electrochemiluminescence immunoassay is intended for use on Elecsys and cobas E-immunoassay analyzers. Clinical outcome was recorded at 1-week and graded according to Modified Rankin Scale.
Statistical analysis
Parametric data are expressed as mean value ± standard deviation (SD) and categorical variables as percentages. The Chi-square test was used for the comparison of dichotomous variables and the Student's t-test for continuous variables. One-way ANOVA was used to test differences on multiple levels by a single factor (independent) variable. P < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS Windows (SPSS version 17.0.2009).
Results
The study included 142 patients with acute stroke who fulfilled inclusion criteria were admitted the MGH attached to Dr. Sampurnanand Medical College Jodhpur. Out of total 80 (56.33) male and 62 (43.66) were female. In addition, 38 healthy volunteers (25 female and 13 females) similar to the patients cohort in term of age and sex formed the central group. The mean ± SD age of the total patients was 63.73 ± 14.11 years and of the control was 57.94 ± 10.66.
Types of strokes and serum S-100 protein level
The most common type of stroke in our study was ischemic (66.19) followed by hemorrhage (24.64) and transient ischemic attack (TIA) (9.15). The most common condition in stroke patients was HTN (68.30), followed by ischemic heart disease (47.8%) diabetes mellitus was seen in 28.87% and dyslipidemia in 23.94%. Seven patients had rheumatic heart disease (RHD) 5%. Serum S-100 protein concentration were increased above the normal value (0.1782 ± 0.1622 ng/ml) in both ischemic (1.12 ± 1.58) and hemorrhage (0.6317 ± 0.782) stroke group which was statistically significant (P < 0.001) when compared to TIA (0.22 ± 0.25) which was not significant (P = 0.23). When within patients group, comparison were made there was a statistically significant difference in serum S-100 protein level between the group according to stroke subtype. Serum S-100 level were significantly higher in the cardioembolic stroke and hemorrhage stroke group than in than TIA group. In our study, serum S-100 level was significantly higher in the ischemic group than in other group of stroke. Atherosclerosis (82.5%) 78 was the most common etiology of the ischemic stroke, followed by ischemic cardio embolism (9.3%) and rheumatic cardio-embolism (8.2%). S-100 protein level maximum for RHD (1.523).
Patients followed by IHD (1.342) and atherosclerosis (1.062) patients [Table 1, Figures 1 and 2].
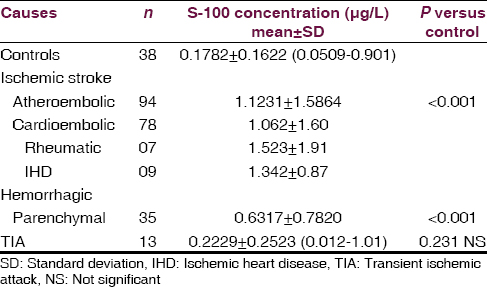
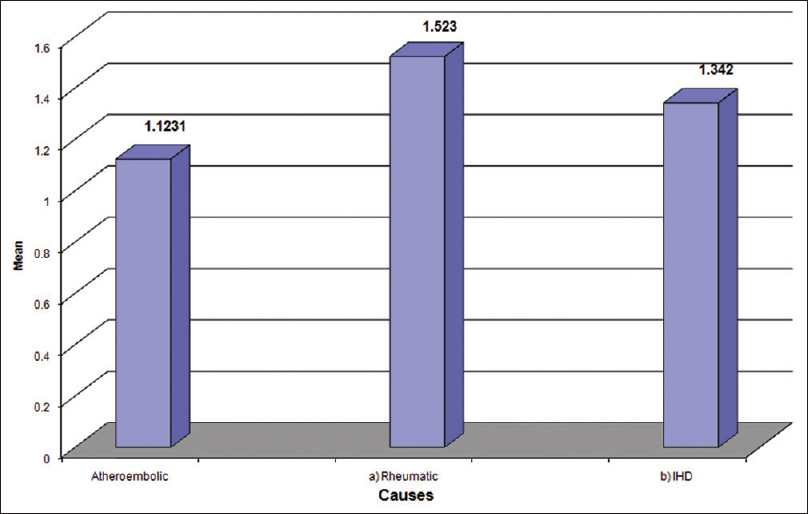
- Serum S-100 protein level in stroke patients
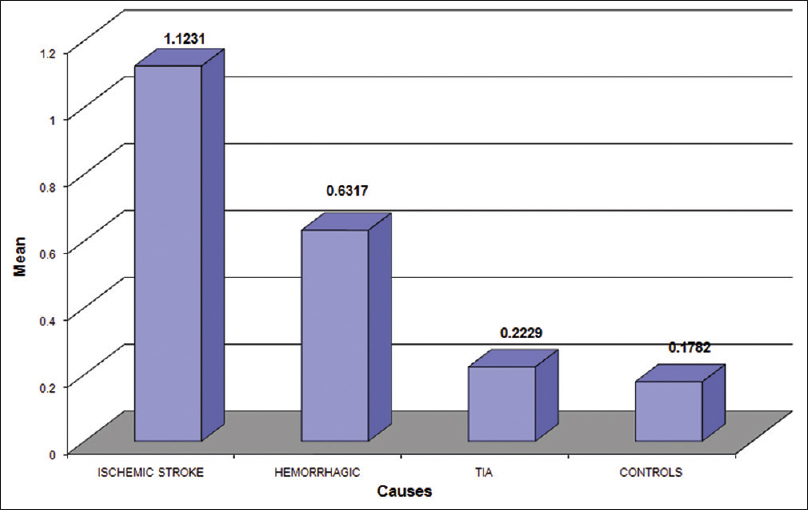
- Serum S-100 protein level in subtype of strok
Severity of stroke and serum S-100 protein level
According to the NIHSS score[14] stroke group were compared with control according to low (NIHSS 1–7) moderate[89101112131415] high (>15) severity stroke had significantly higher serum S-100 protein level (P < 0.001,0.001,0.0001) respectively and S-100 protein level (all comparison P < 0.001), when within patients group comparison were made for stroke severity there was a statistical difference for S-100 protein level were significantly higher in patients with high severity stroke compared those who had low and moderate severity (S-100 median 0.157, 0.270, 1.21). Thus mean S-100 level significantly higher in the stroke patients who belong to NIHSS > 15 [Table 2 and Figure 3].

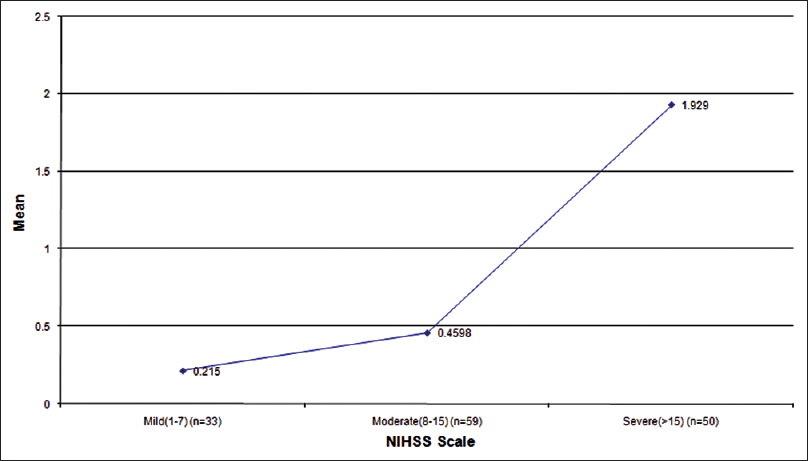
- Serum S-100 level with severity of stroke according to National Institute of Health Stroke Scale scale
Mortality and serum S-100 protein level
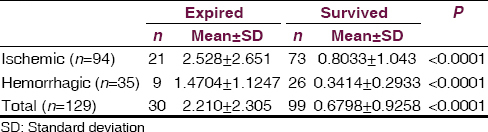
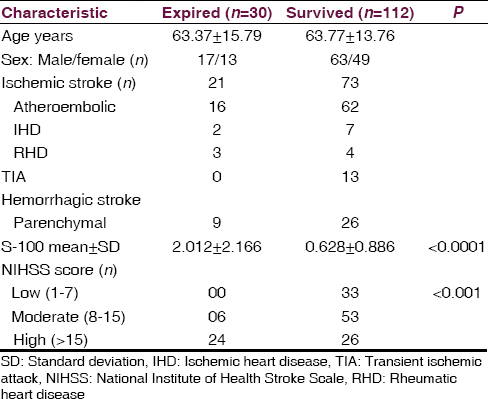
In total, 30 (21.9%) patients died during the study. Demographic and clinical difference between patients who died and those who survived age are given in Table 3. There was a significantly higher value of serum S-100 protein level in patients who expired after stroke than in patients who survive (P < 0.0001). Of the 30 patients who died, 24 patients had NIHSS score ≥15 (P < 0.001). Of the 30 died patients 21 patients was ischemic and 9 patients was a hemorrhagic stroke group and no one died in TIA group. The S-100 protein level was significantly higher in patients who died of ischemic stroke than hemorrhagic stroke [Figure 4, Tables 3 and 4].
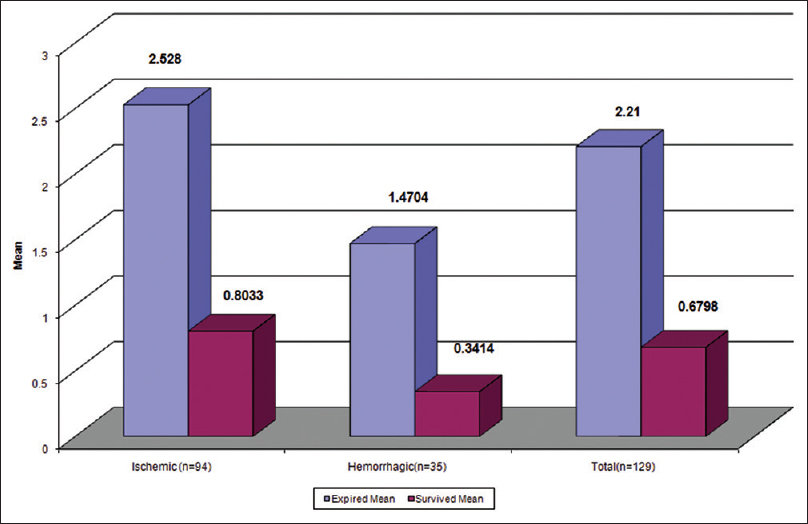
- Serum S-100 level in survived and expired patients
Discussion
S-100 protein is such a neurochemical marker of brain damage which is a major component of the cytosol, particularly in the astroglial cells and is released in the CSF and peripheral blood due to disruption of blood brain barrier after the brain damage. The present study primarily confirmed that S-100 protein level are higher in patients with stroke compared with healthy volunteers. The high level of S-100 protein in acute stroke was also observed with other studies in the literature.[1516] This study also gives the information that S-100 protein level is much higher in ischemic stroke group than other. S-100 protein level was significantly higher in the athero embolic stroke group than in the TIA, cardio embolic and lacunar stroke. S-100 protein level was significantly higher in the patients who died compared with survivors. In predicting stroke subtype and severity although S-100 protein was more valuable for mortality.
An elevated level of S-100 protein was not diagnostic of stroke as it is elevated in all the stroke patients irrespective of types as compared to control. However, S-100 protein can only predict the presence of neuronal damage in the brain but does not specify the cause for it. Hence, S-100 protein has high sensitivity with low specificity in stroke. In other studies also elevated levels was not diagnostic of stroke, the negative predictive value was 0.92.[17] However, it is definitely predicted the prognosis of the patients.
The importance of S-100 protein in acute stroke also confirmed with other studies.[1518] Person et al.,[6] Kim et al.[19] and Missler et al.[20] report an association between single biochemical marker from blood in the acute phase of stroke and functional outcome of infarction.
Foerch et al.[21] measured single S-100 protein in acute stroke within 12–24 h and found it as a valuable marker in guiding clinical and therapeutic aspects in stroke. Herrmann et al.[22] found serum S-100 protein level with the size of the brain lesion and neurological status at discharge. In this study, there is a correlation between serum S-100 protein level, infarct size and outcome.[1723] Kenangil et al.[23] who found that size of the infarct in middle cerebral artery (MCA) territory correlation with the level of S-100 protein. A patient with large MCA infarction had highest serum S-100 level, and their short and long prognosis was the worst. Similar report presented by Buttner et al.[8] Rainer et al.[24] report that within first 24 h there was increased the level of S-100 protein level in stroke patients.
Conclusion
Serum S-100 protein level significantly rises in patient with acute stroke due to ischemia and hemorrhage, but not significant in TIA. It helps in the diagnosis of stroke and the severity of stroke as it is significant increases according to the size of the lesion thus it is more valuable for the prognosis. S-100 protein levels have a positive correlation with NIHSS. Finally, we conclude that serum S-100 protein measurement can be used as an early marker of brain damage. There is a role of S-100 protein as a co-predictor of outcome in patients with acute stroke.
Acknowledgment
Special thanks to Dr. Prakash Choudhary.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- S-100 protein and neuron-specific enolase in CSF after experimental traumatic or focal ischemic brain damage. J Neurosurg. 1989;71:727-31.
- [Google Scholar]
- Neuron-specific enolase and S-100 protein levels in cerebrospinal fluid of patients with various neurological diseases. J Neurol Sci. 1983;60:443-51.
- [Google Scholar]
- Cerebrospinal neuron-specific enolase, S-100 and myelin basic protein in neurological disorders. Acta Neurol Scand. 1995;92:247-51.
- [Google Scholar]
- A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739-44.
- [Google Scholar]
- Serum S100beta: A noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806-13.
- [Google Scholar]
- S-100 protein and neuron-specific enolase in cerebrospinal fluid and serum: Markers of cell damage in human central nervous system. Stroke. 1987;18:911-8.
- [Google Scholar]
- Serum S-100 protein, relationship to clinical outcome in acute stroke. Ann Clin Biochem. 1997;34(Pt 5):546-50.
- [Google Scholar]
- S-100 protein: Serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28:1961-5.
- [Google Scholar]
- Comparison of serum S-100 protein levels following stroke and traumatic brain injury. J Neurol Sci. 2000;181:104-10.
- [Google Scholar]
- Release of neurobiochemical markers of brain damage is related to the neurovascular status on admission and the site of arterial occlusion in acute ischemic stroke. J Neurol Sci. 2004;227:49-53.
- [Google Scholar]
- Association of serial biochemical markers with acute ischemic stroke: The National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508-13.
- [Google Scholar]
- S100B as a surrogate marker for successful clot lysis in hyperacute middle cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 2003;74:322-5.
- [Google Scholar]
- Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62:1130-4.
- [Google Scholar]
- Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521-6.
- [Google Scholar]
- Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology. 2004;63:1317-9.
- [Google Scholar]
- Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology. 2005;65:513-7.
- [Google Scholar]
- Increased serum S-100 protein in patients with acute ischemic stroke; relationshop to clinical outcome. Med Connnect. 2012;7:45-8.
- [Google Scholar]
- Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology. 2007;68:415-9.
- [Google Scholar]
- Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke. 1996;27:1553-7.
- [Google Scholar]
- S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956-60.
- [Google Scholar]
- Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004;35:2160-4.
- [Google Scholar]
- Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670-7.
- [Google Scholar]
- Relation of serum S-100 protein to infarct size and clinical prognosis. Marmara Med J. 2004;17:105-8.
- [Google Scholar]
- Comparison of plasma beta-globin DNA and S-100 protein concentrations in acute stroke. Clin Chim Acta. 2007;376:190-6.
- [Google Scholar]






