Translate this page into:
Pupil to limbus ratio: Introducing a simple objective measure using two-box method for measuring early anisocoria and progress of pupillary change in the ICU
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Measurement of static pupillary size in the ICU is of importance in cases of acutely expanding intracranial mass lesions. The inaccuracies with subjective assessment of pupillary size by medical personnel preclude its use in emergent neurological situations.
Objective:
To determine if the ratio of pupil to limbus diameter (PLD ratio) measured by a two-box method is a reliable measure of pupil size for detecting early anisocoria and measuring pupillary changes.
Materials and Methods:
The PLD ratio was defined as the ratio of the pupillary diameter measured at a para-horizontal axial plane with the limbus diameter measured at the same or parallel axial plane. A two-box method was used to estimate the diameters of imaged pupils. Eyes were imaged using an iPhone 4S cellphone camera. Background illumination was measured and kept constant. The pupils of a 78-year-old woman, who presented with a large intra-axial parenchymal hemorrhage, were imaged. The patient had left pupillary miosis in dark but not in bright light. After presenting this case along with the images of the pupillary examination, a group of 21 medical staff were asked several questions on the pupillary examination. Reliability of PLD ratio were assessed via standard error of mean (S.E.M) of PLD ratios for 3 different subjects each imaged under constant illumination and fixation but from different angles to the optical axis.
Results:
Analysis of questionnaire data together with PLD ratios revealed that ~ 14% and 10% of participants could estimate the pupillary size in darkness and bright light respectively but none were simultaneously accurate indicating that subjective assessment of pupillary size was unreliable. The approach towards a systematic pupillary examination was inconsistent among the participants. The PLD ratio was found to be a reliable measure of pupillary size with standard error of mean below 0.1 mm for the three subjects tested.
Conclusion:
Static pupillary sizes can be objectively and consistently evaluated using PLD ratios using a two-box method. PLD ratios are resistant, within limits, to changes in imaging angle or choice of para-horizontal axes for measurement.
Keywords
Acute intra-cerebral mass lesion
anisocoria
intensive care unit
limbus
pupil
two-box method
Introduction
The pupil, an aperture located in the center of the iris of the eye regulates the entry of light into the retina by the action of two antagonistic muscles in the iris, the sphincter and the dilator. Activation of the sphincter, under parasympathetic control constricts the pupil (miosis), whereas activation of the dilator, under sympathetic control dilates the pupil (mydriasis). The parasympathetic and sympathetic pathways under the influence of complex neuroanatomical pathways control: (1) Pupillary size under conditions of steady background illumination, accommodation and vergence; (2) velocity of change in size with light onset or offset. Both the pupillary size and its velocity of change in size can be affected by several mechanisms such as structural lesions in the brain, disease states and pharmacological agents among others (for details see[1]).
Difference in pupillary diameters between the two eyes measured under conditions of equal illumination, vergence and accommodation are used to characterize anisocoria. Anisocoria with a difference in pupillary diameters greater than 0.4 mm is considered abnormal.[2] Acute anisocoria can occur because of an expanding mass lesion (for example, edema following an acute ischemic stroke,[3] intracerebral hemorrhage[4] or craniocerebral trauma[5] due to either pressure from mass effect or from ischemia.[6] Detection of acute anisocoria is of importance in the ICU because it impacts management of patients with acutely expanding mass lesions. This is because: (a) It is a sign of acute expansion of mass lesion and consequently of raised intracranial pressure;[7] (b) consequent to early detection of acute anisocoria fast intervention either surgically or medically (for example, by hyperventilation and hyperosmolar therapy) is related to improved recovery,[8] (c) its presence is a prognostic indicator of functional recovery (for example, after traumatic transtentorial herniation,[910] (d) the duration of pupillary changes has been found to be tied to survival, for example, in traumatic brain injury, craniotomy for traumatic hematoma done when bilateral pupillary non-reactivity is present for less than but not more than 3 hours was linked with good survival at 1 year.[10]
Abnormal static pupillary size in acutely expanding mass lesions as manifested by anisocoria can be caused by sub-functional parasympathetic or sympathetic centers (for details see).[11] Decreased central sympathetic control which leads to miosis can be due to the involvement of the sympathetic hypothalamic control centers (for example from hemorrhages[12] or infarction[1314] or brainstem lesions,[13] whereas decreased parasympathetic control may be due to transtentorial herniation resulting in compression of the 3rd nerve[1516] or low brainstem oxygenation due to low perfusion/ischemia affecting the occulomotor nucleus.[17]
Inaccuracy in subjective assessment of pupillary size by physicians and nurses[1819] and inaccuracy in repeated subjective evaluations of the pupils by diff erent examiners preclude its use in emergent neurological situations.[18] Subjective assessment of anisocoria has also been found to have low inter-rater reliability within neurologists.[1920] Twa et al. (2004) found that pupil diameter measurements using digital photography were more repeatable than measurements made with other devices such as: Colvard-pupillometer, semicircular templates and millimeter ruler.[21] The digital photographs used by Twa et al. were taken in controlled laboratory conditions with proper alignment, a Nikon 990 digital automatic flash exposure, fixed focus at 17 cm, and maximum optical zoom.[21] In this study we posit that useful pupillary measurements can be made at the bedside using readily available imaging device with autofocus and auto-exposure capabilities as found in cell-phones.
The limbus, which constitutes the border between the white opaque sclera and transparent cornea,[22] has been used for normalizing the pupillary size for each eye in digital photographs in animal studies,[2324] and can practically eliminate the problems of subjective errors, variable alignment, measurement distance, perspective and magnification in the assessment of anisocoria. We propose the ratio of pupil to limbus diameter (PLD ratio) as a robust indicator of pupil size in humans. PLD ratio is defined here as the ratio of the pupillary diameter measured at an axial plane (in this study a para-horizontal axial plane was chosen) with the limbal diameter measured at a same or parallel axial plane. In this study we define a 2-box method for accurately estimating PLD ratios. Using the PLD ratios as reference, we assessed the accuracy of the subjective evaluation of anisocoria within nurses, residents, senior medical students and residents involved in day to day care in the ICU. We also measured PLD ratios in digital photographs taken from various perspectives from the observed eye to evaluate its robustness.
Materials and Methods
Image capture
Prior to imaging, informed consent from the patient and all subjects were taken. In addition, all images were de-identified. Before imaging, the patient and subjects were exposed to the ambient light levels for at least 5 minutes. The camera of a cell-phone was used for imaging (iPhone 4s; Model number: A1387). The metering mode of a camera refers to the way in which a camera determines exposure. We used built-in spot metering capability of the camera, wherein the reflectance of light from the patient's iris/pupillary area was used to automatically adjust for exposure without the use of flash. We also used the built-in auto-focus capability of the camera. Upon directing the camera toward the patient's eyes the region of interest (the pupil/iris) was chosen on the iPhone 4s LCD screen by tapping with the finger on the previewed image of the subjects face being photographed. A light-blue square reticle appeared on the region of interest which pulsated briefly until focus was acquired and appropriate exposure times auto-calculated and auto-adjusted by the camera. The image was then taken.
Illuminance measurement for the ambient light conditions were made using a Luxmeter (Model no. LX 1010B, Mastech Guangdong, China) with a range of 1-50,000Lux, resolution of 1Lux and repeatability of ±2%. The range of illuminance values ranged from 1 to 1035 Lux for Figure 1 a–d (Figure a–b: 1035 Lux; Figure c–d: 1 Lux) and between 665 and 722 Lux for Figure 3 a–i.
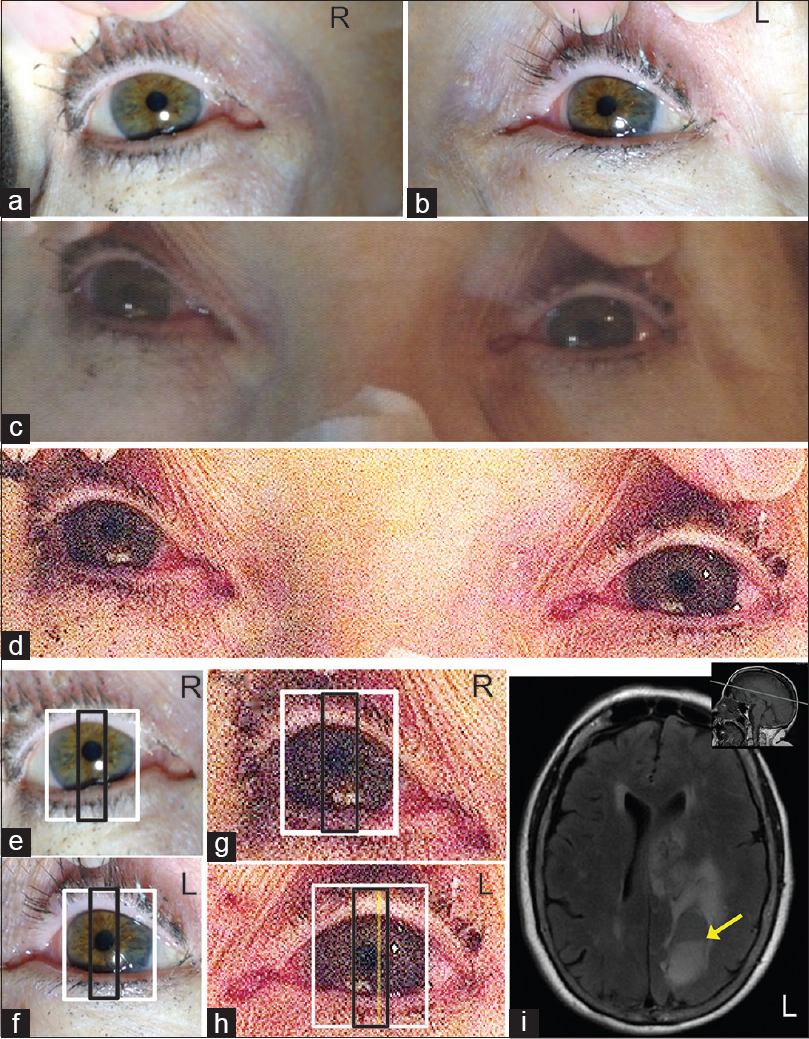
- (a and b) Patients right (R) and left eye (L) imaged under bright light conditions;(c) unprocessed image of patients eyes imaged under dim light conditions;(d)image in C adjusted for brightness and contrast to highlight the pupillary margins in patients eyes.(e-h) Measurement of limbal and pupillary diameters of the R and L eyes imaged under bright (e and f) and dim (g and h) background illumination. The larger white box (e-h) represents the limbus diameter, which was equal in both eyes and measured 11.5 mm. The smaller black box represents the diameter of the pupil measured in the same axial plane as the larger white box. The yellow line in H represents the left edge of the patient's right pupil represented on the left eye. The distance between the yellow line in H and the right-edge of the black box in H represents the extent of anisocoria. There was a difference in PLD ratios of 0.0708 or 0.85 mm. (i) Fluid-Attenuated Inversion Recovery (FLAIR) MRI of brain showed large area of intra-axial parenchymal hemorrhage and surrounding T2 signal hyperintensity on the left. There was mass effect on the cingulate gyrus, corpus callosum and left lateral ventricle. Mild shift of the septum pellucidum to the right by approximately 8 mm was observed. Cystic area with fluid-fluid level (yellow arrow) is noted posteriorly in the left parietal region. Inset, sagittal T1-weighted image, the grey line indicates the axial plane illustrated in the FLAIR image
Image analysis
All images were adjusted for brightness and contrast to clearly delineate the pupillary margins. Figure 1a and b were rotated to align the eyes such that the outer and inner canthi of each were on a horizontal plane. This was done to enable ease of assessment for the participants in the survey. Microsoft Office Powerpoint 2007 (Microsoft Corporation Redmond, WA, USA) was used to measure PLD ratios. The PLD ratios were estimated by a two-box method. Two boxes were drawn using the rectangle tool of the drawing toolbar, such that the heights of the two boxes were equal and superimposed. The widths of the larger box (white box in Figures 1e–h and 3) and smaller box (black box in Figures 1e–h and 3) were adjusted manually so that its width represented the limbal and pupillary diameters, respectively. For each PLD ratio limbus and pupillary diameters were estimated in the same or parallel para-horizontal axis that was visible.
Participants involved in the survey
Participants consisted of neurology and internal medicine residents rotating in the MICU, 4th year medical students, nurses involved in stroke management and paramedic. There were a total of 21 survey participants. Consent was obtained from all participants for an anonymous survey.
The survey participants were given a case-presentation (described below) with images using Microsoft Power Point displayed onto a 40" LCD flat panel display with a 1,280- by 768-pixel resolution (NEC LCD4000) or projected with an image size of 72 inch × 54 inch. The pupillary apertures were clearly visible to all the survey participants.
Case presentation
A 78-year-old right-handed Caucasian woman was at a friend's house when she had difficulty speaking followed by right arm and right leg weakness. On evaluation in the emergency room she was found to have profound expressive aphasia. Her vascular risk factors included hypertension, but she was not taking any medications. She had no known allergies and she did not drink alcohol, smoke tobacco, or use recreational drugs. Vitals on admission were significant only for systolic hypertension (170/80 mmHg). She was awake with sustained eye opening; she had expressive aphasia and could follow simple commands, such as raising her left hand or nodding yes and no appropriately. She had a right facial droop and hemiplegia involving both proximal and distal muscles of the right upper and lower extremities. Routine labs complete blood count (CBC), comprehensive metabolic panel (CMP) and coagulation profile were unremarkable. MRI of patient's brain showed large area of left sided intra-axial parenchymal hemorrhage with mass effect and midline shift to the right [Figure 1i]. The patients static pupillary sizes were imaged under conditions to steady background illumination after light adaptation of 5 minutes, for bright light conditions (~1035 Lux) and dim light conditions (~1 Lux).
Procedure
The survey participants were first presented with MRI images of the brain [Figure 1i]. Subsequently, the participants were shown sequential images of the patient's pupillary exam (see below) and were asked the following multiple choice questions:
-
“Which pupil is more dilated compared to the other?” Figure 1a and b were shown and participants were informed that these images were taken under bright light conditions. The participants were asked to select one of three choices: Left pupil, right pupil or both are the same size
-
“Which pupil is more dilated compared to the other?” Figures 1c and d were shown and subjects were informed that these images were taken under low light conditions. The participants were giventhe same three choices as in 1. The contrast and brightness of the picture in 1cwere optimized to display the pupillary margins in 1d
-
“What kind of illumination would you ideally use for examining the static pupillary size?” The participants were asked to select one or more of the following responses: Bright light, dim light and background illumination should not matter as long as both pupils are visible. Static pupillary examination indicates estimation of pupillary size under conditions of constant background illumination, fixation and vergence
-
“How good are you in identifying sluggish pupils?” The participants were asked to select one of the following responses: Good all the time, good when the pupils are initially dilated, good when the pupils are initially constricted or not good at all
-
“What is your preferred position while examining the patient's pupils at bedside?” The participants were asked to select one or more of the following responses: Usually right side, usually left side, head end of the bed, usually centered to the patient's eyes and foot end of the bed
-
“What source of illumination is best for measuring static pupillary size under conditions of constant illumination?” The participants were asked to select one of the following responses: Pen light with low intensity, pen light with high intensity or uniform room illumination
-
“Does detecting pupillary change affect decision on patient management in stroke cases?” The participants were asked to select one of the following responses: Yes or no.
To investigate if PLD ratios were consistent when measured from various perspective and distances as expected in the ICU where images would be taken by different observers, images of a single eye were taken from three volunteer subjects (aged 29yrs, 61 yrs, 34 yrs) from different perspectives left to right or from center to below, in multiple positions from which both the lateral and medial limbal and pupillary circumferences were visible (~30° from the optical axis of the eye). All images were taken while maintaining constant background illumination, vergence and fixation (at ~8 ft). The PLD ratios for every 5 degree difference in orientation across 35 degrees starting from an available para-horizontal diameter across 6 images for each were also measured. As indicated previously all measurements were taken at constant background illumination for each subject which varied from 665 to 722 Lux. None of the three subjects had pronounced hippus.
Results
The PLD ratios were computed using pupil and limbal diameters obtained from a two-box method as mentioned in the methods section. The results illustrated in Figure 1e–h indicate that in this patient there was anisocoria which was best seen in dim illumination. The left pupil was miosed compared with the right pupil under dim light conditions. There was an interocular difference in PLD ratios of 0.0708 (difference of 0.85 mm). The relative miosis in the left pupil corresponded to the intracerebral hemorrhage seen on left cerebral hemisphere resulting in mass effect on the lateral ventricles and diencehpalic area on the left [Figure 1i].
When survey participants were presented with Figure 1a and b showing the patients pupils recorded under bright light conditions, with two different but equally scaled perspectives viewed from the right or left, most participants (~86%, Figure 2a) incorrectly noted a difference in the pupillary size [see also Figure 1g and h. When presented with Figure 1d showing the patients pupils recorded under dim light conditions, with a frontal view of both eyes, most participants (~90%, Figure 2b) incorrectly noted no difference in pupillary size [see also Figure 1e and f]. Only 28% [Figure 2c] of the participants correctly chose uniform room illumination instead of a directional light source such as a pen-light as the proper illumination for measuring static pupillary size (pupil size under conditions of constant background illumination). About 33% [Figure 2d] of the participants indicated they were good at identifying sluggish pupils, whereas the majority (~57%, [Figure 2d]) felt that they were good at identifying sluggish pupils only if the pupils were initially dilated. About 29% [Figure 2e] of the participants indicated that they centered the patient's eyes (with or without turning the patients face towards them). Further, only 14% [Figure 2f] of the participants correctly stated that pupillary measurements were best performed both in low background and high background illumination. The majority of participants (~95% Figure 2g) agreed that pupillary measurements did impact patient management.
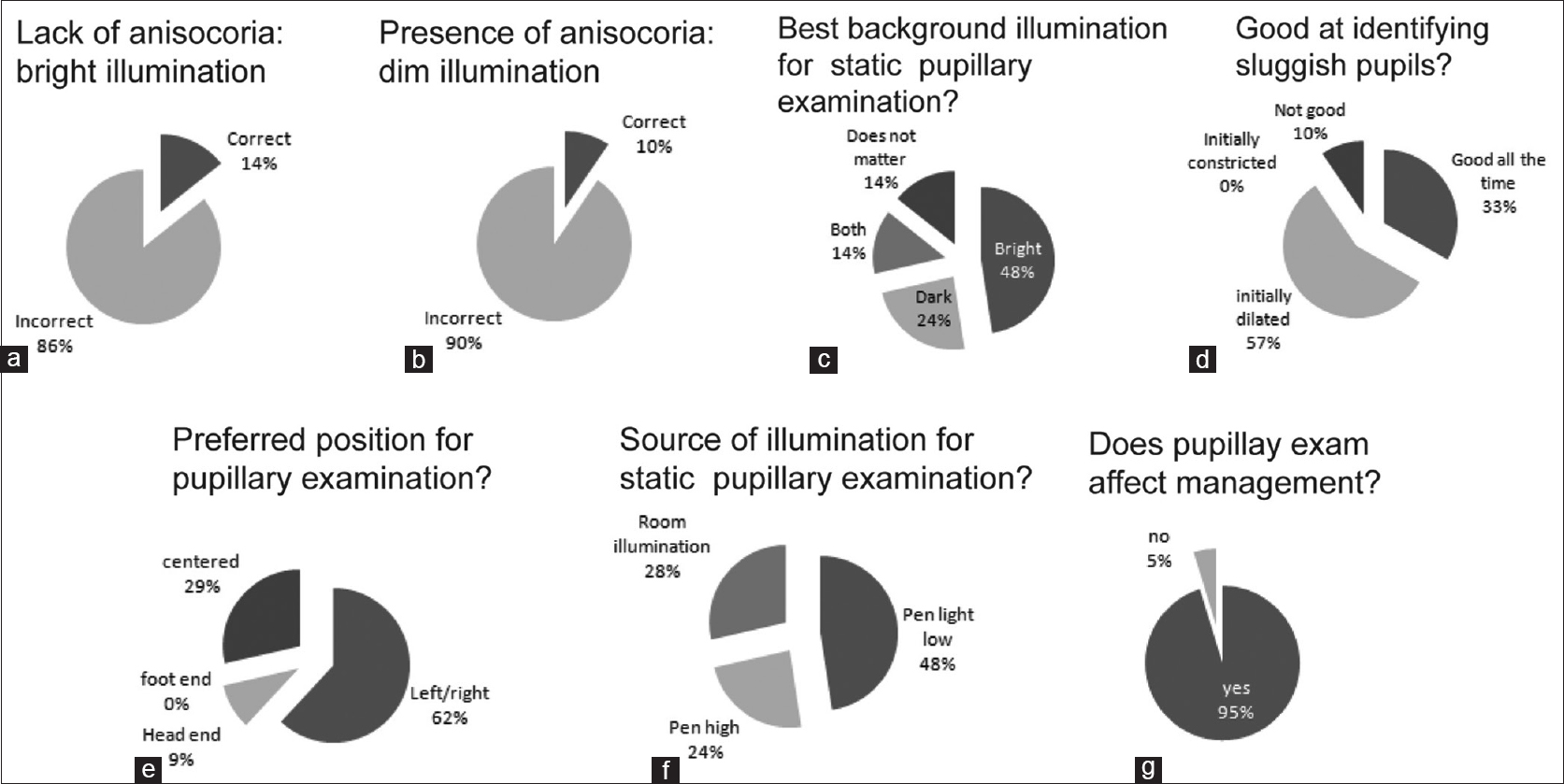
- The results of survey of the participants on the pupillary examination. (a) Question asked: “Which pupil is more dilated compared to the other?” Lack of anisocoria under bright light conditions was the correct response whereas presence of anisocoria was the incorrect response. (b) Question asked: “Which pupil is more dilated compared to the other?” Right pupil more dilated than the left (i.e., left miosis) was the correct response whereas absence of anisocoria was the incorrect response (c) Question asked: “What kind of illumination would you ideally use for examining the static pupillary size?” The choices were: Pupils imaged in darkness, bright light, both or that the background illumination did not matter. To determine a parasympathetic or sympathetic pupillary dysfunction, pupils should be examined both in the dark and under bright light. (d) Question asked: “How good are you in identifying sluggish pupils?” The choices referred to an estimate of dynamic pupillary response to a flash of light for different initial pupillary diameters. The choices were: Pupils initially constricted, pupils initially dilated, the state of pupillary diameter did not matter (good all the time) or not good. (e) Question asked: “What is your preferred position while examining the patient's pupils at bedside?” These choices referred to position of the examiner at the bedside during pupillary examination of a patient laying in bed. Centered implies centering the patients head with respect to the examiner, which could involve orienting the examiner and/or turning patients head. Left/ right, head end and foot end refers to the side of the bed where the examiner positions himself while examining the pupils. Not being centered can cause subjective estimations errors in detecting anisocoria because of the viewing perspective. (f) Question asked: “What source of illumination is best for measuring static pupillary size under conditions of constant illumination?” This question refers to the light source the examiner uses for estimating pupillary size. Room illumination with a diffuse light source is best for examining static pupillary sizes whereas directional light sources such as pen light (either dim or bright) can produce different illumination for each of the two retina resulting in differences in pupillary size. (g) Question asked: “Does detecting pupillary change affect decision on patient management in stroke cases?” The choices were: yes or no
We next investigated if the PLD ratios were accurate when an eye was imaged from various angles and at various distances from the camera. During imaging, the subjects kept their eye fixed at a point. All measurements were done under same conditions of illumination. For each of these images the medial and lateral limbal edges were clearly visible. Some examples are illustrated in Figure 3. The PLD ratios for nine random perspectives tested for three subjects including the ones in Figure 3 were 0.263 (±S.E.M 0.0004), 0.307 (±S.E.M0.0007), and 0.362(±S.E.M0.0008). The PLD ratios for the three subjects for who each eight PLD measurements for every 5 degree difference in orientation across 35 degrees and across six images [Figure 3j–l] were: 0.263 (±S.E.M 0.0003), 0.307 (±S.E.M 0.0005) and 0.362 (±S.E.M 0.0005). The significance of the standard error of mean for the PLD ratios, a measure of variance from the mean, is explained in the discussion.
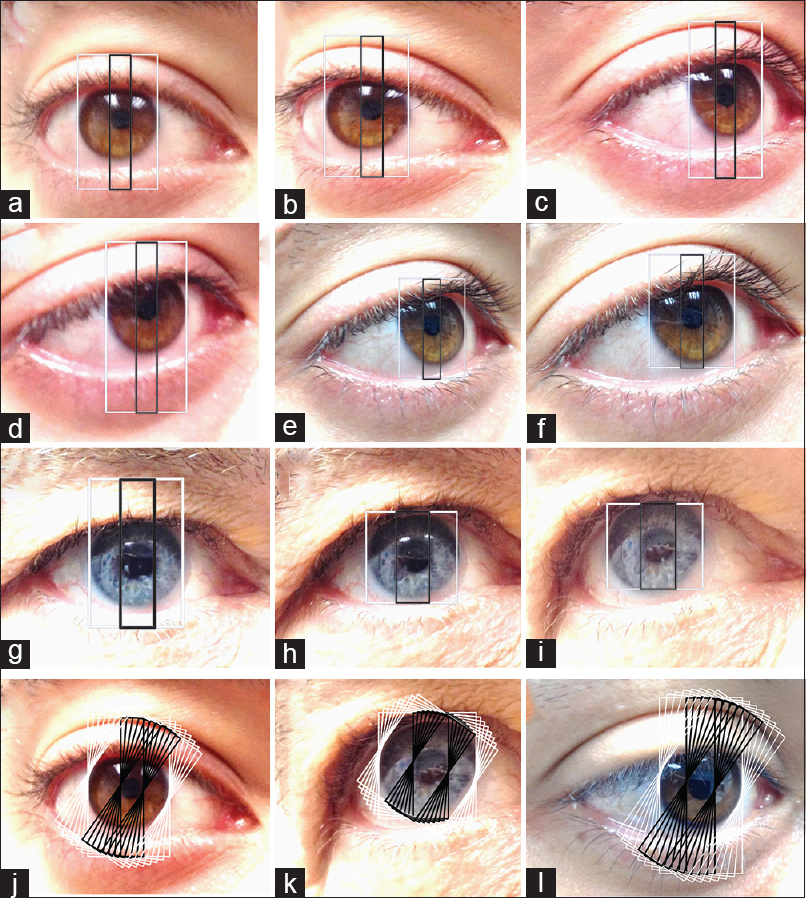
- Examples of the images of three subjects (X,Y,Z): X: (a,b,c,d,e,f), Y (g,h,i) and Z (l) photographed from different angles and different distances. The PLD ratios were computed at para-horizontal axes. The width of larger outer (white) box represents an axial diameter of the limbus, whereas the smaller (black) box represents the axial pupillary diameter measured on the same axis as the limbus. The pupil to limbal diameter (PLD) ratios for ninerandom perspectives tested for the subjects X and Y were 0.263 (±S.E.M 0.0004) and 0.307 (±S.E.M 0.0007), respectively. The pupillary sizes on frontal view were X: 3.024 mm (A,B,C,D,E,F) and Y: 3.53 mm (G,H,I). PLD ratios measured in sample ocular images of subjects X,Y and Z (X:j, Y:k, Z:l) at different para-horizontal axes (eight paired boxes (black + white) each at 5 degree increments over¬ 35 degrees). The PLD ratios for X, Y and Z for eightpaired PLD measurements (for every 5 degree difference in orientation across 35 degrees) across siximages each were X: 0.263 (±S.E.M 0.0003), Y: 0.307 (±S.E.M 0.0005) and Z: 0.362 (±S.E.M 0.0005)
Discussion
The case presented to the participants shows ipsilateral miosis resulting in anisocoria in a patient with intracerebral hemorrhage causing mass effect on the diencephalic area likely involving the hypothalamic sympathetic centers. The anisocoria was measurable under conditions of dim ambient illumination but not under bright light conditions.
This study finds that subjective assessment of anisocoria is unreliable. Only 10% of participants correctly identified the pupillary size equality under the bright light conditions and 14% identified the pupillary differences under the dim light conditions. None of the participants who were correct in identifying the pupils under the bright light conditions were able to do so under the dim light conditions, indicating that these judgments were likely based on chance or confounded by the presence of distracters. Potential distracters in our case were:(1) MRI brain of the patient was shown to the subjects who thus knew the laterality of hemorrhage before assessment of anisocoria (a common situation in the ICU where imaging studies are already available at the time of patient assessment); (2) participants were asked to determine which pupil was “dilated” compared with the other (expectation of a third nerve involvement being a common expectation in the ICU).
The relative constancy of the PLD ratio measurements captured from different perspectives from the imaged eye is also supported by experimental data on the shape and orientation of the pupil and the limbus. Both the pupils and the limbus closely approximate perfect circles.[25] The projections of a circle from eccentric perspectives as captured by images are ellipses. The ellipses originating from pupil and the limbus of an eye for practical purposes can be considered as scaled version of the other sharing parallel major and minor axes.[25] It is important to note that the major and minor axes are parallel but may not be in the same line [Figure 4]. This is because although the pupil and the limbus appear to share the same orientation the pupil is slightly eccentric with respect to the limbus[25] and this eccentricity changes with pupillary size under various conditions of illumination.[26] Thus measuring the pupilary diameter in the same axis as the limbus will give an erroneous result as illustrated in Figure 4. The two-box method of estimating the limbal and pupil diameters (see Methods) circumvent this problem by measuring the pupillary diameters in parallel axes.
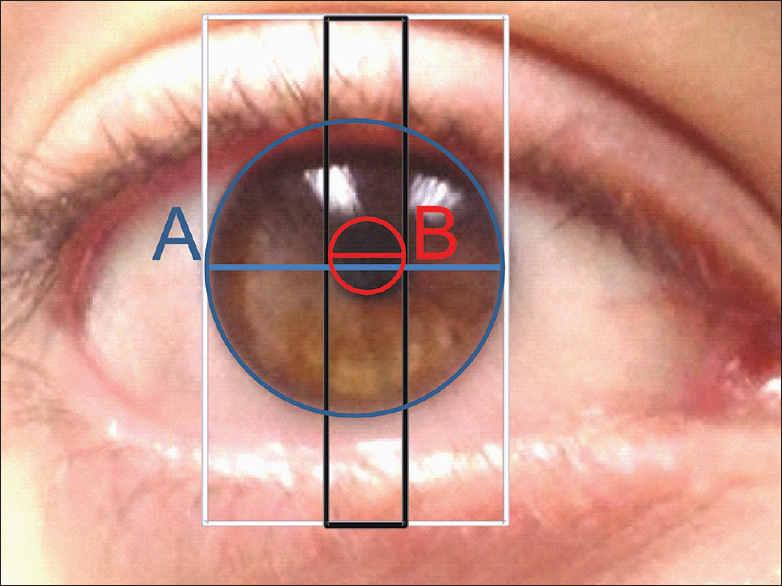
- The box method for estimation of PLD ratios measures limbal diameter (A, blue diameter) and pupil diameter (B, red diameter) pupillary sizes at parallel axes and not always the same axes. The separation between the two diameters is proportional to the pupillary eccentricity
The PLD ratio of different subjects taken from the different angles and at different para-horizontal axial diameters (see methods section) were very similar with a low S.E.M (max S.E.M was 0.0008), indicating that these measures were reliable. For a 11.5 mm limbus diameter, pupillary measures between 0.1 and1mm translate to a PLD ratio in the range between 0.009 and 0.087, this range is substantially greater than the S.E.Ms obtained by PLD measurements from digital photographs. Thus, pupillary images can be captured easily at the bedside by different caregivers virtually eliminating inaccuracies in diagnosis of anisocoria that may arise from examiner bias and perspective. The PLD ratios measured at different para-horizontal diameters for each image gave very similar ratios.
For all images taken at each ambient light levels using the cameras spot-metering capability, we found that the digital camera gave identical F-stop, ISO speed and exposure times. We also found that the brightness values registered by the camera for calculating exposure had very little variation and ensured the exact exposure setting for all images taken under each conditions of background illumination. These brightness values can be used for cross-reference on the ambient lighting conditions helping in documentation.
Microsoft Powerpoint was used for image analysis because this was readily available. Any graphing software including the graphing tools that are provided by most electronic medical records system (once images are uploaded) can be used for the two-box method of estimating PLD ratios. Because the contrast between the cornea versus sclera and iris versus pupil is relatively high, this two-box method can be easily automated to estimate PLD ratios. Pupil to limbal area instead of pupil to limbal diameter can give the same PLD ratio, but will not be able to give a value for relative eccentricity of the pupil if that is additionally required.
One limitation of our study was that we did not image the pupils for illuminance below 1 lux. The image resolution for conditions below 1 lux was fuzzy. The lowest illumination tested, at which anisocoria was found in the patient [Figure 1c and d] was in the mesopic range at which both rods and cones are active in the retina.[2728] Additionally, we did not measure dynamics of pupillary changes. Alternative image capture paradigms in the dark such as use of flash photography or IR illumination with an IR capable camera were also not employed by this study. The presence of significant hippus can confound static pupillary measurements. In the presence of significant pupillary or corneal asymmetries PLD ratio measurements will have limited utility in the detection of anisocoria but can be used for plotting the time course of pupillary changes.
In summary, the PLD ratios are an objective measure for rapidly and accurately quantifying anisocoria and following the time course of change of the pupil in the ICU. PLD ratios can be reliably estimated using the two-box method from digital photographs that can be taken from a readily available cellphone camera that has spot-metering capability at no additional cost. Evaluation of pupillary size via PLD ratios should include imaging under conditions of darkness and bright light to addresses involvement of the parasympathetic or sympathetic control centers in acute brain lesions. Keeping the camera angle not too far from the optical axis, using spot-metering and avoiding superior views gives well-defined limbal and pupillary edges for measuring the PLD ratios. The patient's eyes should be fixed at a target, if the patient is conscious, to eliminate the effects of accommodation and vergence on the pupillary size. Future studies should focus on development and testing of portable hand-held methodologies to rapidly measure PLD ratios for a range of illuminations as well as measure PLD ratios dynamically.
Source of Support: Nil
Conflict of Interest: None declared.
References
- The Pupil. Anatomy, Physiology and Clinical Applications. Vol 1. Detroit, Michigan: Wayne State University Press; 1993. p. :1106-8.
- [Google Scholar]
- The Human Eye: Structure and Function. Sunderland, Massachusetts: Sinauer Associates; 1999.
- [Google Scholar]
- The behr pupil revisited. Anisocoria following cerebrovascular accidents. Stroke. 1975;6:697-702.
- [Google Scholar]
- Rapid expansion of hypertensive intracerebral hemorrhage. Neurosurgery. 1992;31:35-41.
- [Google Scholar]
- The localizing value of asymmetry in pupillary size in severe head injury: Relation to lesion type and location. Neurosurgery. 1994;34:840-6.
- [Google Scholar]
- Prognosis and clinical relevance of anisocoria-craniotomy latency for epidural hematoma in comatose patients. J Trauma. 1996;41:120-2.
- [Google Scholar]
- Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int. 2011;2:82.
- [Google Scholar]
- Fixed and dilated pupils after trauma, stroke, and previous intracranial surgery: Management and outcome. J Neurol Neurosurg Psychiatry. 2001;71:175-81.
- [Google Scholar]
- Functional recovery after traumatic transtentorial herniation. Neurosurgery. 1991;29:227-31.
- [Google Scholar]
- One-year outcome following craniotomy for traumatic hematoma in patients with fixed dilated pupils. J Neurosurg. 1995;82:961-5.
- [Google Scholar]
- Adler's Physiology of the Eye. Philadelphia, Pennsylvania: Elsevier Health Sciences; 2011. p. :502-525.
- [Google Scholar]
- Horner's syndrome due to hypothalamic infarction. Clinical, radiologic, and pathologic correlations. Arch Neurol. 1986;43:199-200.
- [Google Scholar]
- Infrared pupillometry during uncal herniation. J Neurosurg Anesthesiol. 2002;14:223-8.
- [Google Scholar]
- Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 1999;44:941-8.
- [Google Scholar]
- Inter-observer variation in the evaluation of neurological signs: Patient-related factors. J Neurol. 1994;241:492-6.
- [Google Scholar]
- Influence of additional information on interrater reliability in the neurologic examination. Neurology. 1992;42:2076-81.
- [Google Scholar]
- Estimation of pupil size by digital photography. J Cataract Refract Surg. 2004;30:381-9.
- [Google Scholar]
- Muscarinic receptor antagonist and an alpha-adrenergic agonist are required in combination to provide stable mydriasis following intravitreal injection in mice. Biol Med (Aligarh). 2010;2:17-23.
- [Google Scholar]
- Topical mydriatics affect light-evoked retinal responses in anesthetized mice. Invest Ophthalmol Vis Sci. 2010;51:567-76.
- [Google Scholar]
- The Perspective Geometry of the Eye: Toward Image-Based Eye-Tracking, Human-Centric Machine Vision. 2012. InTech. ISBN: 978.953.51.0563.3 Available from: http://www.intechopen.com/books/human-centric-machine-vision/the-perspective-geometry-of-the-eye-towardimage-based-eye-tracking
- [Google Scholar]
- Pupil location under mesopic, photopic, and pharmacologically dilated conditions. Invest Ophthalmol Vis Sci. 2002;43:2508-12.
- [Google Scholar]
- Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036-44.
- [Google Scholar]
- Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol. 2008;586:2551-80.
- [Google Scholar]






