Translate this page into:
Comparative analysis of clinical and computed tomography features of basal skull fractures in head injury in southwestern Nigeria
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Basal skull fractures (BSF) in head injury may be missed clinically. Early detection ensures prompt treatment and prevention of complications We compared the clinical and Computed Tomography (CT) features of basal skull fractures in head injured patients in a southwestern Nigerian hospital.
Materials and Methods:
Head injury patients who had cranial CT at a Southwestern Nigerian hospital were selected. CT images were acquired with a 64-slice Toshiba Aquillion CT scanner using a standard head protocol. The images were evaluated for evidence of skull fractures, and associated complications. The clinical data and CT findings were analyzed.
Results:
One hundred and thirty patients were evaluated, including 103 (79.2%) males. Their ages ranged between 7 months and 81 years, mean 35 years (SD, 20.3). In 59 patients (45.4%, 59/130) BSF was detected on CT, while 71 (54.6%) had no evidence BSF. Forty-two (71.2%) of the 59 patients detected on CT had clinical suspicion of BSF (P < 0.001) while the remaining 17 (28.8%) were not clinically diagnosed. This equaled a sensitivity of 71.2% and, specificity of 90.1% for clinical determination of BSF in this study. There was no statistically significant difference between clinical and CT diagnosis (P > 0.05). The commonest observed clinical feature in patients with confirmed BSF was otorrhagia (45.8%) and the petrous temporal bone (45.8%) was the most commonly fractured bone. The BSF was caused most commonly by motor bike accidents in 53 (40.8%). The most common associated intracranial injuries were intracerebral haemorrhage (34.6%) and subdural (17.3%)
Conclusion:
It appears that neurosurgical evaluation is comparatively reliable in evaluating basal skull fractures in this study area even as they are consistently demonstrated by high resolution CT scanners. A clinical suspicion of BSF should warrant a closer detailed CT evaluation and reporting by radiologists.
Keywords
Computed tomography basal skull fractures
head injury
Nigeria
Introduction
Basal skull fractures (BSF) are serious injuries resulting from a break in the bones of the skull base. These fractures are often associated with dural tears. These may result in cerebrospinal fluid (CSF) fistula presenting as rhinorrhea and otorrhea of which meningitis is a known complication.[12] Other complications of BSF include extra-axial and intra-axial hemorrhage and cranial nerves (CN) injury.[3] Uncomplicated skull fractures themselves rarely produce neurologic deficit, but the associated intracranial hematoma may cause raised intracranial pressure and have serious neurologic sequelae.
The estimated incidence of basal skull fracture from non-penetrating head trauma in the developed world ranges between 7% and 15.8% of all skull fractures, with associated cerebrospinal Fluid (CSF) leakage occurring in 2% to 20.8% of patients.[4] The incidence in Nigeria has not been fully determined. However, BSF was detected in 33% of all head injured patients in a small study by Adeleye et al. in Lagos Nigeria.[2] Clinical signs that may lead a physician to suspect a BSF can be deduced from the part of the skull base that is fractured. Anterior skull base fractures may produce CSF rhinorrhea,[5] rhinorrhagia, periorbital ecchymosis and anosmia, whereas middle cranial fossa fractures may cause CSF otorrhea, otorrhagia, facial nerve palsy,[6] hemotympanum or tympanic membrane perforation with blood in the external auditory canal, retromastoid bruising (Battle's sign), hearing loss, and evidence of vestibular dysfunction. Fractures of the posterior cranial fossa may lead to coma, difficulty in phonation, aspiration and ipsilateral motor paralysis of the vocal cord. However, there may be overlap of these signs: CSF otorrhea and rhinorrhea are common to all parts of the skull-base since dural tear can occur in any of these compartments.
Basal skull fracture may not be clinically apparent at initial clinical evaluation; hence, detection of fractures at CT evaluation may require the need for close observation of the patients for signs of complications of such fractures. Some of these complications are post-traumatic meningitis, anosmia, hearing loss and neurovascular injury. There may be a significant risk of injury to the internal carotid artery when fracture lines extend to the carotid canal. Such injuries may involve compression by hematoma, arterial wall contusion, carotico-cavernous fistula, vascular dissection, false or true aneurysm and occlusion.[7]
High resolution multi-detector computed tomographic (MDCT) scans with multi-planar reconstruction and 3-D surface rendering have dramatically improved the radiological diagnosis of BSF. Moreover, the usual streak artifacts (due to beam hardening) and partial volume averaging seen in the posterior fossa with conventional CT scanners are reduced to negligible proportion in these scanners. In addition, associated intracranial injuries are well depicted on CT scan and acquisition of images is faster. Thus, CT is usually preferred to magnetic resonance imaging (MRI) in an emergency setting for head injury patients. Also, CT scanners are now relatively more widely available in major cities in our country of practice, Nigeria.
Using our 64-slice MDCT scanner as the “gold standard” this study proposes to assess the accuracy of clinical evaluation in predicting the presence of BSF in head injury patients in Nigeria's largest teaching hospital. We also evaluated the pattern of associated intracranial injuries in the setting of BSF in this study cohort.
Materials and Methods
Over a 6-month period from April to October 2013, we prospectively reviewed the brain CT scans of all eligible head injured patients for presence and pattern of BSF at the University College Hospital (UCH), Ibadan in southwestern Nigeria. All consenting head injury patients were included while uncooperative/restless patients whose images showed significant distortions or other artifacts were excluded. Ethical approval for the study was obtained.
All patients were scanned using a 64-slice Toshiba CT scanner with the scan plane parallel to the orbitomeatal line. Helical scans of the cranial base were generated with a 1.5 pitch, 1-mm reconstruction interval, and 1-mm collimation (120 kVp, 220-300 mA) from the C1/C2 level to the vertex. For cases requiring contrast, 40 ml of intravenous bolus of a 300 mg/ml iopromide solution (ultravist) was given and a second series of images obtained. Image reconstruction was pre-determined by the scanner manufacturer and includes filtered back projection with an iterative bone processing filter for the axial image set and a 180° linear interpolation for helical section reconstruction.
The images were evaluated by two radiologists (EO and OGI) on a standard DICOM workstation for evidence of BSF using a bone window algorithm and filter where necessary.
The clinical information was obtained from the case files, in each case after a neurosurgical clinical evaluation at the Emergency Department. The findings were analyzed using the statistical package for social sciences (SPSS) version 20.0 (SPSS, Chicago, IL, USA). Chi-square test was used to test associations between qualitative variables with a P value of 5% considered to be statistically significant.
Results
A total of 130 eligible head injury patients were evaluated. They comprised of 103 (79.2%) males and 27 (20.8%) females with a male to female ratio of 3.8:1. Their ages ranged between 7 months and 81 years with a mean of 34.85 (SD, 20.48).
In this study population head injury (HI) occurred most frequently in the first four decades of life and were least common beyond the seventh [Figure 1]. The most common cause of HI was motor bike road traffic accidents (MBRTA) accounting for more than two-fifths (40.8%) of the injuries (25.4%); motor vehicular traffic accident (MVRTA) (29.2%), and fall from height (20.8%). Assault, gunshot, and occupational hazard were the other infrequent causes of head injury [Table 1]. More specifically, fall from height was the commonest cause of injury in the < 21 year age-group while MBRTA and MVRTA were most common in the 21-30 year category. The most common cause of injury among patients with BSF was MBRTA.
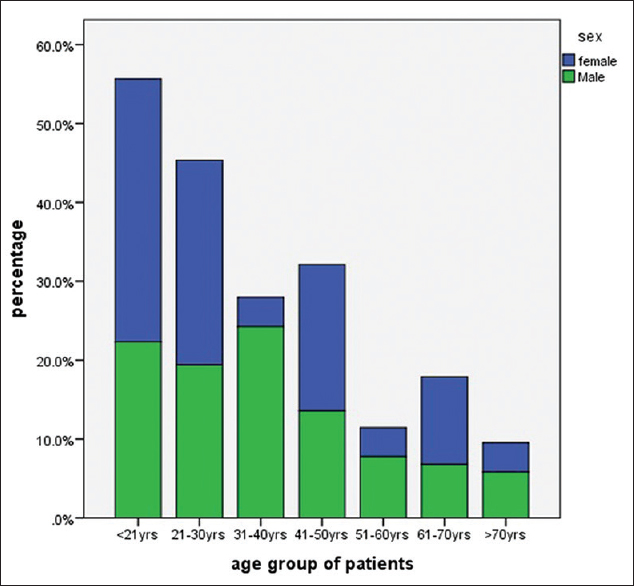
- Age distribution of head injury patients

Of the 130 patients, 59 (45.4%) had BSF at CT examination while 71 (54.6%) had no CT evidence of BSF. Forty-two (71.2%) of the 59 patients were clinically suspected to have BSF (P = 0.001, χ2 = 51.60) while 17 (28.8%) were clinically unsuspected but discovered on CT. There was clinical suspicion of BSF in 7 (1%) of the 71 patients for whom CT revealed no evidence of BSF. This gives a clinical sensitivity of 71.2%; specificity of 90.1%; positive predictive value of 85.7%, and negative predictive value of 79.0%.
The modal age group for patients with BSF was 21-30 years with a male to female ratio of 4.9:1.
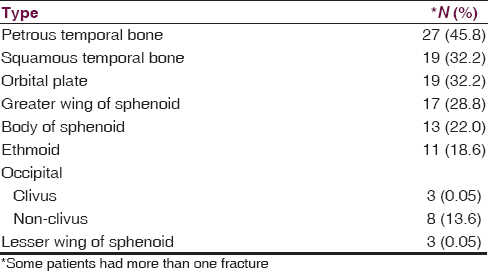
The most common BSF site in this study was the petrous temporal bone found in 27 (45.8%) patients [Figure 2]. The distribution of other fractures is shown in Table 2.
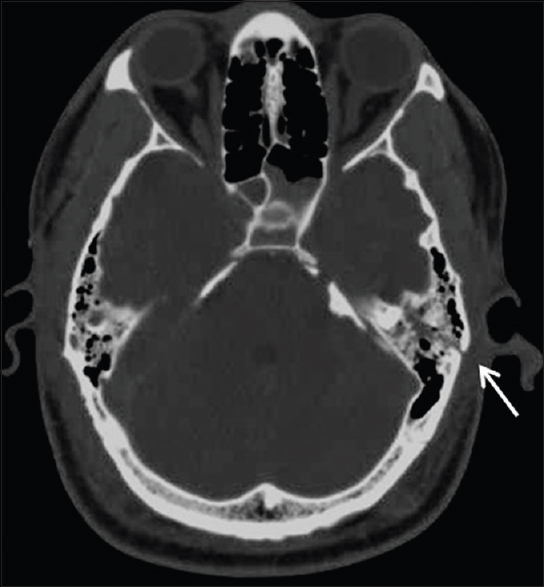
- Axial CT image showing a longitudinal fracture of the left petrous bone (arrow)
Using the traditional classification schema[8] the temporal bone fractures were longitudinal in 15 (55.6%) cases, transverse in 5 (18.5%), and of mixed types in 4 (14.8%). Three patients (11.1%) had longitudinal and transverse fractures involving the petrous temporal bones bilaterally.
The most commonly fractured bone in unsuspected cases of BSF is the squamous temporal bone (37%) followed by the orbital plate of the frontal bone and the greater wing of the sphenoid, which have equal frequencies, (23.5%). The petrous temporal bone was involved in only one unsuspected case.
The most commonly associated intracranial injury in patients with BSF was contusional hemorrhages seen in 29/59 (49.2%) patients. The other associated injuries include subdural hematoma (39%), extradural hematoma (23.7%), and subarachnoid hemorrhage (28.8%). Intracerebral hematoma and intraventricular hemorrhage were also seen with BSF occurring in 16.9% and 18.6% of patients respectively. Compared to the patients without BSF, these associated findings were found more frequently in those with BSF (P < 0.05) [Table 3].
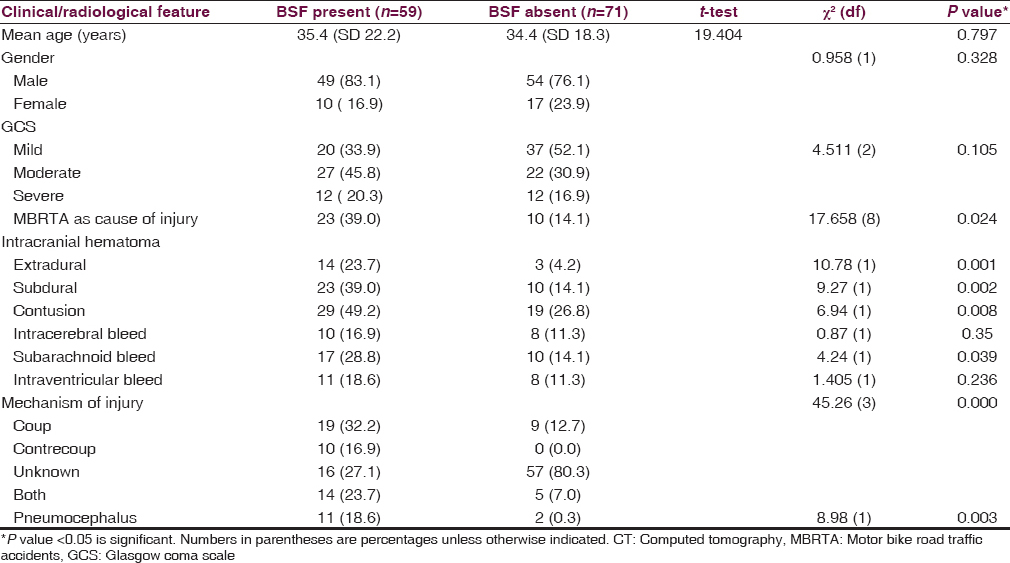
The mechanism of injury was uncertain in majority of the cases [73 (56.2%]. However, more than one in five cases [28 (21.5%)] resulted from a coup mechanism, while the contrecoup mechanism of injury accounted for 7.7% (10/59) and both mechanisms were reported in 19/130 (14.6%) cases. Coup injuries occur at the site of impact, i.e. the intracranial hemorrhage being on the same side as the fracture or soft tissue swelling. The contrecoup injury occurs at the opposite side or at the rebound site of impact. Among patients with CT diagnosis of BSF, the coup mechanism was the most common (32.2%) and both mechanisms of injury were present in a significant number of patients (23.7%).
The clinical predictors in the 59 patients with confirmed BSF at CT were as follows: Otorrhagia 27 (45.8%), rhinnorrhagia 17 (28.8%), periorbital ecchymosis 12 (20.3%), otorrhea 2 (0.04%) and rhinorrhea 1 (0.02%). Retromastoid bruising was not demonstrated in any of the patients.
Of the 17 patients with BSF confirmed only at CT, 3 had clinical signs that could have suggested a BSF, and these were: Rhinorrhagia, CN VII palsy and periorbital ecchymosis. Thirteen (76.5%) of these patients had cranio-facial injuries which may explain why the BSF were clinically missed.
Discussion
Basal skull fractures are recognized complications of head injury, especially following road traffic accidents and falls.[24] The estimated incidence of BSF from non-penetrating head trauma in the west varies between 7% and 15.8% of all skull fractures.[4] Clinical diagnosis of BSF is usually based on the efflux of cerebrospinal fluid (CSF) or blood from the ear, nose or throat. Several studies have shown the significant propensity of post-traumatic meningitis occurring in patients with BSF.[2910] Most of these studies were based on the need for prophylactic antibiotics in such patients to prevent the potentially lethal complication of BSF. However, a few patients may have BSF without dural tear and hence may be difficult to detect clinically.
The sensitivity of clinical evaluation in determination of BSF in Nigeria has not been previously assessed. Hence, there is paucity of regional data on the comparison of clinical features of BSF with CT findings. Since, CT is gold standard for base of skull evaluation and is readily available in Nigeria, we sought to determine the sensitivity of clinical evaluation in predicting BSF.
Our study revealed a re-assuringly high sensitivity of the clinical evaluation for BSF [Table 3]. This may be attributed to the reliability of the time-tested clinical signs. Moreover, the clinical signs were elicited by experienced neurosurgical residents. Thus, in other low income settings with no CT facility, good neurosurgical evaluation can be reliable. Pretto et al. in a study of 142 patients with selected clinical signs of skull base fractures (blepharohaematoma, Battle's sign and bloody otorrhea) reported the positive predictive value (PPV) of these clinical signs in patients with BSF.[11] Battle's sign and unilateral blepharohematoma (periorbital ecchymosis) had 100% PPV for BSF while bilateral periorbital ecchymosis and bloody otorrhea (otorrhagia) had 70% PPV. They suggested that all patients with the selected clinical signs should have cranial CT with bone window to be able to demonstrate the associated BSF. The high predictive values are probably due to the fact that the study was carried out only on patients with signs of BSF which may constitute a selection bias. We on the other hand included all head-injured patients in our study. In another study by Savastio et al., rhinorrhea, otorrhea, focal neurologic signs and Battle's sign demonstrated high predictive values (100%) for intracranial sequelae.[12]
Fracture of the skull base usually occurs in the context of significant head trauma.[8] The most common cause of head injury in this study was road traffic accidents. This is not unexpected as motor vehicles and motorcycles are the prevalent mode of transportation among the working population. Furthermore, rapid motorization, poor road network and poor compliance with traffic safety regulations play a key role in road crashes which account for 70% of all head injury cases in Nigeria.[131415]
We also noted that fall from height, accounted for one-fifth of cases and were mostly among patients younger than 21 years. This suggests negligence and poor supervision of the younger ones by responsible adults. Adeleye et al., in the same setting however previously reported a much lower incidence of 11.2%.[13] No obvious reason was found responsible for this.
The majority of the patients in this study were males with a male to female ratio of 3.8:1. This is likely due to cultural factors in this locality in which more men tend to go out to source for income for their families. Emejulu et al. reported a similar ratio of 3:1 while Adeleye et al. had a slightly higher ratio of 5.8:1.[1315] This may be due to the fact that the latter study was done in a more cosmopolitan city (Lagos) where more men tend to drive vehicles and ride motor bikes. Mohammadi in Iran also documented a similar finding in which more men were involved in RTA (male to female ratio 5:1).[16]
The majority of the patients in this study were adults with more than 75% being over 21 years of age. This age range is similar to other published works from Nigeria and across the globe.[131417] Although the majority of our study subjects were mildly head injured (43.8%) about 46% out of the 59 patients with BSF had moderate head injury. This is probably because patients with BSF usually have associated intracranial injury and were more likely to have suffered more severe trauma.
In all, BSF were present in 45.4% of the patients with head injury in this study. This high percentage of BSF is probably due to the fact that most of the patients had RTA and had sustained serious injuries. This finding is similar to a report by Emejulu et al. in which BSF were found in 43.7% of patients[15] which was somewhat higher than the 33% proportion reported by Adeleye et al.[2] This is possibly due to the fact that the latter study was carried out over a slightly shorter duration and in a slightly earlier epoch. Moreover, the diagnoses of BSF were made in that study based only on clinical suspicion with or without radiologic confirmation in both cases. In all, this comparatively high prevalence rate of BSF in head injuries in these regional data sources (33-46%) is at variance with the finding of Connor et al. in London in which BSF constituted a striking low proportion of all cranial CT evaluated (9%).[7] In other similar developed countries, the incidence is also purportedly low ranging between 3.5% and 24%[7] This may be due, in these advanced countries, to the presence of more favorable transportation demography. These include superior road networks and compliance of the road users with safety regulations like the use of seatbelts and restraints for motor vehicle occupants, and helmets for motorcycle riders.
A previous study by Goh et al.[17] showed clinical signs to be present in 76% of patients with BSF, similar to our finding of 71.2%. In view of this close correlation, CT findings may be used to predict the presence of BSF. However, in the study by Connor et al., only 47% of patients had these signs.[7] No obvious reason was found responsible for this. The most common sign in this study was otorrhagia (45.8%). Retromastoid bruising was not demonstrated, most probably because of our dark-skinned patients.
Otorrhagia and rhinorrhagia were more common reflecting the severity of the causative trauma in Nigeria where most motor cyclist do not wear helmets, and the use of seat belts by vehicle occupants is also not well entrenched. In one of the patients, the BSF was so traumatic that patient not only had the above two findings but also expelled brain tissue from the nose and mouth.
Fractures of the anterior and middle cranial fossae were more common in this study most likely because of the tendency to fall forward or to the side following road traffic injuries. Moreover, the middle cranial fossa is the weakest of the 3 cranial fossae as it consists of thin bones and multiple foramina. The occipital bone is infrequently fractured likely due to its thickness and tendency for falling forward in road traffic injuries. Adeyinka et al. reported similar findings in a recent study.[18] However, Emejulu et al. reported fractures of the anterior skull-base to be more common.[15]
BSF, if untreated is a major cause of meningitis, some authors have advocated for the use of prophylactic antibiotics in the care of the patients concerned. However, others have contested its use because even though the breech in the dura exposes the meninges to nasopharyngeal organisms, meningitis does not develop in every patient with BSF and prophylactic antimicrobial therapy holds the more harm-than-good potential to actually expose the nasopharyngeal and/or the intracranial space in such individuals to more virulent organisms.[2] Several studies have been done on this controversial subject worldwide but there is yet to be a final consensus.[910]
However, patients with BSF that are not given antimicrobial prophylaxis should be placed on close monitoring with a goal of early clinical diagnosis of meningitis. Patients with BSF and associated other intracranial surgical masses such as extradural or subdural hematoma are given appropriate emergency neurosurgical operative interventions to address the lesions. Such prompt treatments have been shown to demonstrate an improvement in the morbidity and mortality of head-injured patients with BSF.
Earlier reports on the intracranial CT findings in head injured patents in Nigeria found subdural hematoma or cerebral contusions to be most common.[1920] Our findings were consistent with these previous reports.
Conclusion
Neurosurgical evaluation for basal skull fractures, in Ibadan, is comparatively reliable as are high resolution multi-detector CT scanners. Males are five times more at risk of BSF than females. Younger adults as well as victims of MBRTA also have a comparatively higher risk than others for BSF. Radiologist should pay closer attention to the base of skull in clinically suspected cases and provide explicit fracture details to the clinician.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Rhinosurgical concept in management of fronto-basal defects with cerebrospinal rhinorrhea. Laryngorhinootologie. 1998;77:264-71.
- [Google Scholar]
- Basilar skull fracture: Outcome of acute care without antibiotic prophylaxis in a Nigerian neurosurgical unit. Turk Neurosurg. 2010;20:430-6.
- [Google Scholar]
- Traumatic cerebrospinal fluid fistulas. In: Winn HR, ed. Youmans Neurological Surgery (5th ed). Philadelphia: W.B. Saunders Co; 2004. p. :5265-72.
- [Google Scholar]
- Imaging of skull base cerebrospinal fluid leaks in adults. Radiology. 2008;248:725-36.
- [Google Scholar]
- Mastoid bone fracture presenting as unusual delayed onset of facial nerve palsy. Am J Emerg Med. 2008;26:386.e1-2.
- [Google Scholar]
- The contribution of high-resolution multiplanar reformats of the skull base to the detection of skull-base fractures. Clin Radiol. 2005;60:878-85.
- [Google Scholar]
- A comparison of temporal bone fracture classification systems. Clin Otolaryngol. 2006;31:287-91.
- [Google Scholar]
- Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev 2006 CD004884
- [Google Scholar]
- Antibiotic prophylaxis after basilar skull fractures: A meta-analysis. Clin Infect Dis. 1998;27:364-9.
- [Google Scholar]
- Positive predictive values of selected clinical signs associated with skull base fractures. J Neurosurg Sci. 2000;44:77-83.
- [Google Scholar]
- Cranial trauma: The predictability of the presentation symptoms as a screening for radiologic study. Radiol Med. 1991;82:769-75.
- [Google Scholar]
- Clinicoepidemiological profiles and outcomes during first hospital admission of head injury patients in Ikeja, Nigeria. A prospective cohort study. Neuroepidemiology. 2009;32:136-41.
- [Google Scholar]
- Etiology of head injuries in southwestern Nigeria: A public health perspective. Internet J Epidemiol 2005:2.
- [Google Scholar]
- Head trauma in a newly established neurosurgical centre in Nigeria. East Cent Afr J Sur. 2008;13:86-94.
- [Google Scholar]
- Road traffic crash injuries and fatalities in the city of Kerman, Iran. Int J Inj Contr Saf Promot. 2013;20:184-91.
- [Google Scholar]
- Is routine computed tomographic (CT) scanning neessary in suspected basal skull fractures? Injury. 1997;28:353-7.
- [Google Scholar]
- Computerized tomography assessment of cranial and mid-facial fractures in patients following road traffic accident in South-West Nigeria. Ann Afr Med. 2012;11:131-8.
- [Google Scholar]
- Radiological evaluation of head trauma by computer tomography in Ibadan, Nigeria. West Afr J Med. 1999;18:33-8.
- [Google Scholar]
- Cranial computed tomography scan findings in head trauma patients in Enugu, Nigeria. Surg Neurol Int. 2011;2:182.
- [Google Scholar]






