Translate this page into:
Occult falcine meningioma unmasked following nearly complete hemorrhagic transformation with resultant spontaneous acute interhemispheric subdural hematoma
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Sudden-onset monoplegia with features of vomiting and headache usually signals an intracranial cerebrovascular event. We describe a 62-year-old man in whom this presentation was the result of the rare occurrence of an almost complete hemorrhagic transformation of a falcine meningioma with resultant acute interhemispheric subdural hematoma, and discuss the risk factors and possible mechanisms that may lead to such an event. The need for careful examination of the available radiology and aggressive tumor removal is stressed.
Keywords
Falcine meningioma
interhemispheric subdural hematoma
monoplegia
Introduction
Benign intracranial tumors, with exception of pituitary adenomas, rarely present with a hemorrhagic event. While hemorrhage in most intracranial tumors like gliomas and metastatic lesions will present as intracerebral hemorrhage, the peculiarity of hemorrhagic transformation in meningiomas is that such hemorrhage may be intratumoral, intracerebral, subarachnoid, or even subdural in nature.
Case Report
A 62-year-old, normotensive and non-diabetic retired school teacher presented with complaints of sudden-onset weakness of right lower limb, urinary incontinence, and severe holocranial headache of 3 days duration. There was no preceding history of trauma; nor was there any history of consumption of antiplatelets or anticoagulants. On examination, he had grade 4/5 power in the right upper limb, but his right lower limb was completely paralyzed. The right plantar was extensor. There was no cranial nerve dysfunction. Bilateral disc margins were blurred.
Computed tomography (CT) scan of brain showed an acute interhemispheric left-sided subdural hematoma with maximal thickness in the left frontal parafalcine region [Figure 1]. The lesion was having a hypodense area inside it and was focally compressing the medial frontal lobe anteriorly. This finding, along with absence of history of trauma, prompted a magnetic resonance imaging (MRI) to be done. This revealed a predominantly hyperintense lesion in T1 and T2 sequences suggestive of subacute blood with localized area of compression on the brain on medial frontal lobe and corpus callosum [Figure 2]. Routine blood investigations revealed normal bleeding, clotting, prothrombin time, and activated partial thromboplastin time with normal platelet levels.

- Non-enhanced computed tomography scan of brain showing an acute interhemispheric left-sided subdural hematoma with maximal thickness in the left frontal parafalcine region with a hypodense area inside it focally compressing the medial frontal lobe anteriorly
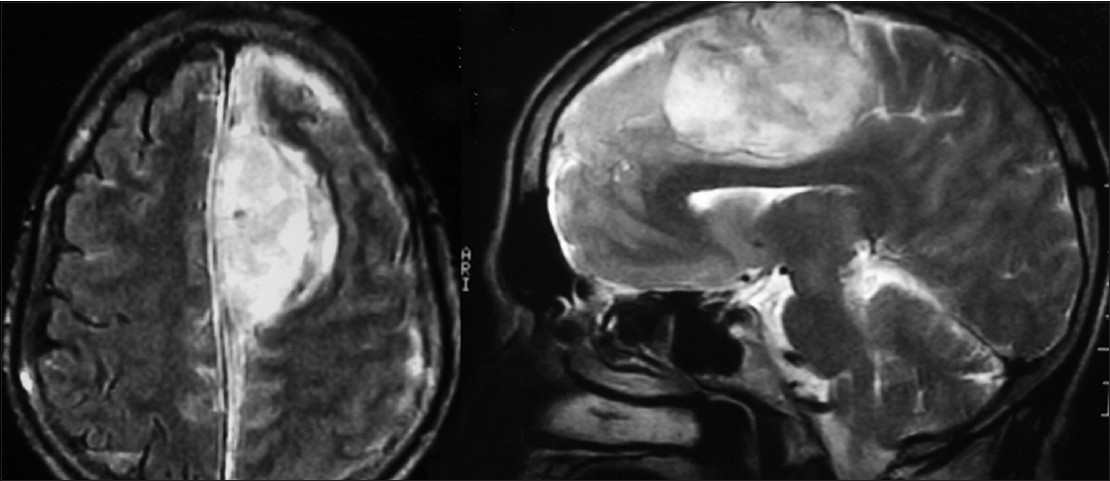
- Magnetic resonance imaging brain showing a predominantly hyperintense extra-axial lesion in T1 and T2 sequences suggestive of subacute blood with localized area of compression on the brain on medial frontal lobe and corpus callosum
The patient underwent a left fronto-parietal parasagittal craniotomy (the day following admission), and as the subdural hematoma was being evacuated, a mildly vascular, grayish lesion was encountered which was adherent to the falx from which it was removed piecemeal after extensive cauterization. No active source of bleeding could be found. The surface of brain was not involved and appeared grossly normal. The specimen was sent for histopathology and was reported as fibroblastic meningioma. The patient had a complete recovery, and contrast-enhanced MRI scans done 1 year later showed no residual lesion [Figure 3].

- Postoperative contrast-enhanced axial magnetic resonance imaging scan showing no residual lesion
Discussion
Around 1.5-5.4% of intracranial tumors present following hemorrhage.[1] Among meningiomas, only 1.3% are reported to show hemorrhage, and this risk is higher in angioblastic and malignant meningiomas.[23] However, none of the 313 patients with meningiomas reported by Cushing and Eisenhardt or the 280 cases of parasagittal meningiomas reported by Hoessly and Olivecrona presented with hemorrhage.[4]
Though meningiomas are commoner in women, no sex predilection has been found in meningiomas that undergo hemorrhage.[4] While some authors hold that location has no bearing on the development of a hemorrhagic event, others hold that cerebral convexity tumor increases the risk of hemorrhage.[3] Helle et al.[4] calculated a “relative bleeding tendency” of meningiomas and concluded that the greatest tendency to bleed was in intraventricular meningiomas. They also reported that the least risk was in transitional and syncytial meningiomas while the maximal tendency for bleed was in the malignant variants.
Various theories have been put forward to explain the genesis of spontaneous hemorrhage in meningiomas, such as predisposition of thin-walled tumor vessels to rupture, blood vessel erosion by tumor, rupture of bridging veins, tumor necrosis following rapid growth of tumor or after cortical/venous sinus thrombosis.[123] Cerebral edema and venous obstruction due to the meningioma can also cause tumor infarction with hemorrhage.[4]
If the bleeding is confined within the tumor capsule, intratumoral hemorrhage is the result. On the other hand, if the hemorrhage spills out of the confines of the tumor, the patient might present with subarachnoid hemorrhage, intracerebral hemorrhage, or subdural hemorrhage. While our patient had presented with subdural hemorrhage, Helle et al. reported that patients may present with more than one type of hemorrhage, the commonest location being subarachnoid (35%). Only 4 of their 43 patients had a pure subdural location of the bleed. Chen et al.[5] also stated that the symptomatic meningioma bleed most commonly occurs in the subarachnoid plane and seldom occurs in the subdural plane. This is surprising as the location of meningiomas is essentially subdural.
The clinical presentation of patients following hemorrhagic transformation in meningiomas is not always acute. Only 14 of 33 (42%) in Helle's series[4] (for whom information was available) had a sudden onset of symptoms. On the other hand, our patient presented in an apoplectic manner. Slow onset may reflect smaller quantities of bleed or may be due to increased brain compliance as a result of preexisting compression by the meningioma.
All authorities stress on surgery with clot evacuation and tumor excision as the treatment of choice. Helle et al.[4] highlighted that all (100%) the 10 cases that underwent only clot evacuation or conservative treatment died as opposed to 7 of the 23 (30.4%) patients who underwent hematoma and tumor removal. Vij et al.[1] mentioned the need for “early diagnosis and adequate treatment” and quoted a study that has described the decrease in mortality rates from 21.1 to 13.9% with availability of CT scanning.
The identification of a previously unsuspected meningioma, like in our case, in the setting of a nearly complete hemorrhagic transformation may be difficult. Worm et al.[3] stressed the need to look for bony changes, areas of focal cerebral distortion and edema near the clot, as well as differences in Hounsfield scale in densities in various regions of the clot. In our case, a hypodense area of tumor in the clot prompted us to consider presence of some other pathology and proceed for an MR imaging. This, in turn, helped to plan the subsequent treatment.
Conclusions
In conclusion, hemorrhagic transformation of a meningioma, though exceedingly rare, may present with sudden deficits (mimicking a stroke) and may unmask a previously occult lesion. Secondly, though the hemorrhage in the meningioma may spread to various planes, we feel the uncommon subdural location probably has good long-term prognosis rather than the intracerebral variant that may give rise to fixed deficits. Further, one must look carefully for density differences in the acute hemorrhage as well as appearance of the overlying skull, as these may be the only indicators of a meningioma in the presence of a hyperdense clot. Early surgery with the aim of removing the clot and the tumor in a single-stage operation is likely to yield the most optimal results. Finally, while literature states that bleeding is commoner in angioblastic and malignant meningiomas, this may not always be the case and every attempt must be made to remove all visible tumors to obviate recurrence.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Meningioma with hemorrhagic onset: Two case reports. J Cancer Res Ther. 2012;8:145-7.
- [Google Scholar]
- Subdural hemorrhage associated with falcine meningioma. Neurol India. 2003;51:419-21.
- [Google Scholar]
- Subdural hematoma in a patient with meningioma. Arq Neuropsiquiatr. 2009;67:308-10.
- [Google Scholar]
- Haemorrhage associated with meningioma: A case report and review of the literature. J Neurol Neurosurg Psychiatry. 1980;43:725-9.
- [Google Scholar]
- Unsuspected meningioma presenting as a subdural hematoma. J Neurol Neurosurg Psychiatry. 1992;55:167-8.
- [Google Scholar]






