Translate this page into:
A young patient with multisystem complications after cytomegalovirus infection
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We are describing a case of an 18-year-old male patient with cytomegalovirus (CMV) associated guillain-barre syndrome (GBS) who presented with an acute onset of generalized weakness and numbness in the extremities, dysphagia, and facial diplegia, followed by respiratory failure, which led to mechanical ventilation. He had positive immunoglobulin G and immunoglobulin M antibodies against CMV, and CMV polymerase chain reaction was positive with <2000 copies of deoxyribonucleic acid. Human immunodeficiency virus test was negative. He received a course of ganciclovir, intravenous immunoglobulin, and plasmapheresis. After improving from acute episode, patient was transferred to a rehabilitation facility for physical and occupational therapy. At the rehabilitation facility, he exhibited signs of acute abdomen with pain in the left upper quadrant secondary to peritonitis from dislodged gastrostomy tube and underwent exploratory laparotomy. During the hospital course he was found to have splenic infarct and colitis on the computed tomography of abdomen. This case showed an immunocompetent young patient with multisystem complications including guillain-barre syndrome (GBS), splenic infarct, hepatitis, and colitis due to CMV.
Keywords
Cytomegalovirus
guillain-barre syndrome
hepatitis
splenic infarct
Introduction
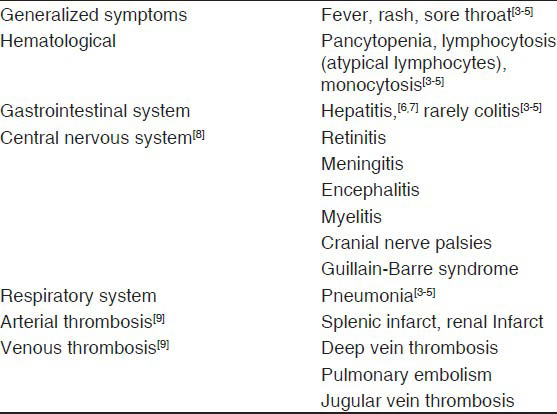
Cytomegalovirus (CMV) associated guillain-barre syndrome (GBS) accounts up to 15% of all GBS cases.[1] The clinical manifestations of GBS associated with CMV occur often after respiratory tract symptoms, preferentially affects young women (<35 years), and are frequently associated with sensory loss.[2] The clinical presentation of acute CMV infection include infectious mononucleosis syndrome (i.e., fever, rash, sore throat, atypical lymphocytes in the peripheral blood, monocytosis, thrombocytopenia, and elevated liver enzymes), pneumonitis, retinitis, colitis, and any other organ involvement.[345] CMV infection may rarely present with severe organ specific complications in immunocompetent hosts. The frequent presentation is severe hepatitis.[67] The second frequent complications in immunocompetent patients are central nervous system disorders such as meningitis, encephalitis, myelitis, nerve palsies, and GBS.[8] The cases involving multiple systems due to CMV are very rare. We are describing a case of CMV-induced hepatitis, colitis, splenic infarct, and GBS in an 18-year-old male with intact immune status.
Case Report
An 18-year-old previously healthy male presented to the clinic with the complaints of sore throat and generalized malaise. The patient denied any history of alcohol or drug use. He denied any history of recurrent infections. The patient had no history of bleeding diathesis. His platelet count was normal. He had a negative rapid monospot and rapid strep antigen test. He recovered from acute illness in 3 days.
He noticed dysphagia and paresthesias of both lower extremities and weakness in all extremities (lower > upper, proximal > distal) one day after recovering from sore throat. On neurological examination, patient had bilateral facial nerve palsy and bulbar palsy. Motor strength was grade 1 in lower extremities and grade 2 in upper extremities. Muscle tone of the limbs was decreased. Deep tendon reflexes on the upper limbs were diminished and absent on the lower limbs.
Blood chemistry was normal, except for total bilirubin of 2.3 mg/dL, alanine transferase and aspartate transferase levels of 87 IU/L and 67 IU/L respectively, indicating hepatitis, which may be due to acute CMV infection. Test for human immunodeficiency virus was negative. His serology was positive for CMV immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies. CMV polymerase chain reaction (PCR) was positive with <2000 copies of deoxyribonucleic acid (DNA). Cerebrospinal fluid analysis was done, and it was positive for CMV IgG antibody and increased amount of proteins. He was treated with ganciclovir for acute CMV viremia and received three doses of intravenous immunoglobulin without much response. Patient had diffuse decrease in sensory and motor amplitudes, low normal conduction velocities, prolonged distal motor latencies in the left upper limb, and absent or prolonged F-wave responses on nerve conduction studies with severely decreased recruitment throughout, and a few positive sharp waves and fibrillation potentials on needle electromyography. These findings were most consistent with an acute diffuse neuropathy, most likely acute inflammatory demyelinating polyradiculopathy in early stage.
Day 4: Patient developed acute respiratory failure and was placed on mechanical ventilator.
Day 12: He demonstrated a slow and gradual improvement in his strength mostly in upper extremities after starting plasma exchange daily for 5 days.
Day 15: He was discharged to a rehabilitation facility. He had bifacial weakness; motor strength was 2/5 in lower and 3/5 in upper extremities. Sensations were intact.
Day 16: He developed acute onset of abdominal pain in left upper quadrant with high grade fever (103.4 F) and was admitted to the hospital. He was found to have dislodged gastrostomy tube, which was replaced by the interventional radiology. After that, the patient had an exploratory laparotomy. He was started on broad-spectrum antibiotics and culture was sent which grew coagulase negative staphylococcus. The patient continued to have high-grade fevers (102.9 F) despite exploratory laparotomy and aggressive wash out. Repeat CMV PCR was negative. Serum IgG ganglioside GQ1b antibody was <1:100 titers.
Day 21: Repeat computed tomography (CT) abdomen showed modest sized splenic infarct [Figures 1 and 2] and prominent colitis. Patient had a small hypodense focus in the periphery of the spleen on the follow-up CT abdomen on day 25 and day 32, which was consistent with evolution of splenic infarct.
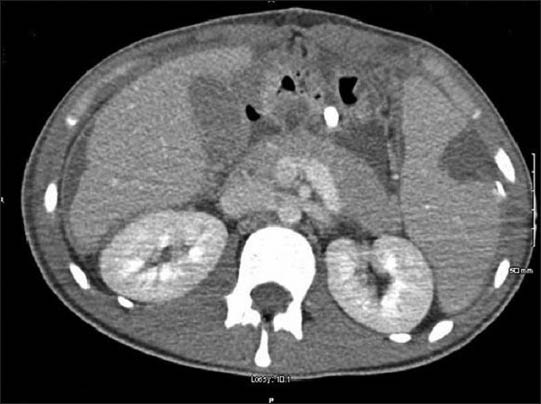
- Horizontal section of CT abdomen showing splenic infarct
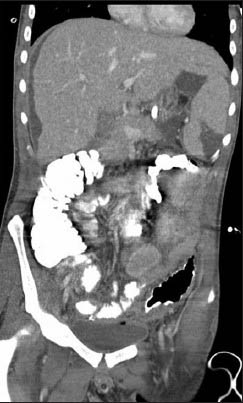
- Coronal section of CT abdomen showing splenic infarct
Day 42: CT abdomen on the day of discharge showed decrease in the size of splenic infarct. Patient's condition was improved gradually and ultimately he was discharged to a rehabilitation facility.
Discussion
The clinical features of CMV infection in immunocompromised hosts have been extensively reported, but comparatively less attention has received for those observed in immunocompetent patients. Table 1 describes the clinical manifestations of CMV infection in immunocompetent patients. The diagnosis of acute CMV infection is based on positive CMV-specific IgG and IgM antibodies, peripheral blood positive for CMV pp65 antigens, and ≥600 copies of CMV DNA/mL.[9]
Arterial or venous thrombosis in patients with acute CMV infection is not rare and is independent of the typical risk factors for thrombosis. In a retrospective study by Atzmony et al.,[9] the incidence of thrombosis was 6.4% (n = 9) in 140 patients with acute CMV infection. Splenic infarcts (n = 4) and renal infarct (n = 1) were the manifestations of arterial thrombosis. Venous thrombosis was presented as pulmonary embolism (n = 1), lower limb deep vein thrombosis (n = 1), upper limb deep vein thrombosis (n = 1), and jugular vein thrombosis (n = 1).
GBS is an acute demyelinating polyneuropathy that can occur after an infectious disease accounting for 60-70% of cases with CMV infection.[10] Neurological involvement by CMV is usually attributed to aberrant immunological responses triggered by CMV.[11] The outcomes have significantly improved in GBS patients with management in intensive care unit on ventilator support, the use of intravenous immunoglobulin, and plasmapheresis.[12] The diagnosis of CMV associated GBS was based on the presence of clinical features of acute onset of generalized weakness and numbness, which progress rapidly, together with facial diplegia and bulbar palsy; the cytoalbumino dissociation on cerebrospinal fluid analysis; the demyelinating motor and sensory neuropathy on electromyography; and serological evidence of increased IgM and IgG antibodies against CMV. Our patient was unique in that he developed splenic infarct and colitis after the initial episode of GBS and hepatitis.
In the prospective cohort study by Orlikowski et al.,[2] sensory deficits were observed in 72% of cases and facial palsy in 49% of cases, and test results positive for CMV DNA in 62% of cases tend to be associated with objective sensory defect (P = 0.052). The main factors associated with long-term neurological deficits (21%) were older age (P = 0.001) and assisted ventilation during hospitalization (P = 0.005). Our patient had sensory deficits, positive for CMV DNA, and required the mechanical ventilation.
Two case reports highlighted the acute CMV patients presenting with bilateral abducens palsy.[1314] In another case report patient had GBS along with periodontitis which may serve as a reservoir for CMV and its replication.[15] Sinardi et al.,[16] reported an uncommon case of isolated vasculitis, restricted to the left sylvian artery in a patient with an autoimmune CMV associated GBS.
Conclusion
Multisystem complications can occur after CMV infection even in immunocompetent patients. Early recognition of complications is important and prompt treatment is critical to improve overall outcome. We cannot rule out a rare hereditary immunodeficiency in our patient. However, it is unlikely as the patient was healthy in the past.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Cytomegalovirus infection and Guillain-Barre syndrome: The clinical, electrophysiologic, and prognostic features. Dutch Guillain-Barre Study Group. Neurology. 1996;47:668-73.
- [Google Scholar]
- Guillain-Barre syndrome following primary cytomegalovirus infection: A prospective cohort study. Clin Infect Dis. 2011;52:837-44.
- [Google Scholar]
- Cytomegalovirus infection in immunocompetent and immunocompromised individuals: A review. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:179-87.
- [Google Scholar]
- Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol J. 2008;5:47.
- [Google Scholar]
- Cytomegalovirus induced hepatitis in an immunocompetent host. Mymensingh Med J. 2008;17:S104-6.
- [Google Scholar]
- Acute cytomegalovirus hepatitis in immunocompetent host. Kathmandu Univ Med J (KUMJ). 2009;7:79-81.
- [Google Scholar]
- Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 1997;24:52-6.
- [Google Scholar]
- Incidence of Cytomegalovirus-associated thrombosis and its risk factors: A case-control study. Thromb Res. 2010;126:e439-43.
- [Google Scholar]
- Guillain-Barre syndrome: Epidemiology, pathophysiology and management. Drugs. 2004;64:597-610.
- [Google Scholar]
- A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barre syndrome. Dutch Guillain-Barre Study Group. N Engl J Med. 1992;326:1123-9.
- [Google Scholar]
- Guillain–Barre syndrome and disturbance in multiple organs associated with cytomegalovirus infection. No To Shinkei. 1990;42:245-51.
- [Google Scholar]
- Bilateral abducens palsy in a case of cytomegalovirus-associated Guillain-Barre syndrome. Neurol Sci. 2011;32:1219-22.
- [Google Scholar]
- Cytomegalovirus-associated periodontitis and guillain-barre syndrome. J Periodontol. 2005;76:2306-11.
- [Google Scholar]
- A case of central nervous system vasculitis related to an episode of Guillain-Barrè syndrome. Crit Care. 2000;4:245-7.
- [Google Scholar]






