Translate this page into:
Aneurysmatic bone cyst of the craniocervical region: Surgical technique
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aneurysmal bone cysts (ABCs) are nonneoplastic bone lesions that may originate of any site of skeleton. The spine can be affect up to 30% of the cases, leading to pain, neurological deficits, and pathological fractures in symptomatic patients. The incidence of craniocervical (occipito-C1-2) occurrence is not known. We describe the surgical technique and clinical results of two patients with craniocervical ABCs that underwent primary surgical resection: An 11-year-old girl with a lesion in the posterior aspect of the atlas, and a 28-year-old man with an important hydrocephalus and a posterior expansible lesion on the left side of his posterior fossa. Total resection was achieved on both lesions, with no surgical morbidity. Even though ABCs are nonneoplastic lesions, subtotal resection is associated with early recurrence. The knowledge of the anatomy of the region in order to achieve the occlusion of arterial feeders prior to surgical resection itself is the key point of the surgical strategy.
Keywords
Aneurysmal bone cysts
craniocervical junction
nonneoplastic bone lesion
Introduction
Aneurysmal bone cysts (ABCs) are benign highly vascularized nonneoplastic osseous lesions associated with severe intraoperative bleeding.[12] These lesions are composed of multiple thin-walled blood-filled cysts with a rich vascular array. They are responsible for bone destruction and neural compression secondary to expansion of the cortical limits of the compromised bone.[3]
ABC may originate at any site in the skeleton, but most often they affect the metaphysis of long bones. The spine can be affected in up to 30% of the cases.[2] The expansion of the bony elements may cause cord compression, progressive neurological deficits, pain, and pathological fractures.
The pathogenesis is probably attributed to an initial bone injury, such as a local trauma, leading to an intraosseous arteriovenous communication. This arteriovenous shunt finally results in high-pressure vessels eroding the bony trabeculae, forming a cystic cavity that expands the cortical bone.[3]
In about 20-30% of the cases, ABC is associated with other lesions such as fibrous dysplasia, giant cell tumors, and osteoblastomas. In those cases, some authors prefer to use the term − secondary ABC.[4] The diagnosis of ABC can be performed using computed tomography (CT) scan, which defines the extension and the site of bone involvement. Multiple cavities containing different amounts of fluid are common findings. Also, a CT angiogram could bring additional information about the arterial supply. Magnetic resonance imaging (MRI) is useful to determine the extension of soft tissue involvement and allows visualization of the compressed neural tissue. Those are the most important preoperative diagnostic procedures.[3] An additional digital angiogram can also be necessary to identify the arterial supply when the CT angiogram is not conclusive. In this paper, we describe the surgical technique and results of two patients with craniocervical ABC confirmed by histopathology analysis that underwent primary surgical resection.
Case Report
Case 1
An 11-year-old girl presented in our outpatient clinic with history of an expansible mass and local pain at the dorsal portion of her neck for the last 4 months. A CT scan was compatible with an intrinsic bone lesion, with multiple cysts, in the posterior aspect of the atlas [Figure 1]. No neurological compromise was noticed. A CT angiogram demonstrated that the tumor was highly vascularized. The child was taken to surgery. No prior embolization was performed. The patient was positioned prone using a head holder. With a midline incision, the lesion was completely dissected from the superficial layers of skin. A subperiostal dissection was then performed, exposing all the tumor limits [Figure 2a]. The arterial supply of the tumor came from both occipital arteries that were occluded at the beginning of the dissection [Figure 2b]. The tumor was then removed in small portions. After total removal, we could clearly see both C1 lateral masses and the dura mater exposed [Figure 3a]. The total surgical bleeding estimated was 200 mL.

- Illustrative case − Patient 1-An 11-year-old girl presented in our outpatient clinic with history of an expansible mass and local pain at the dorsal portion of her neck for the last 4 months (a and b) Computed tomography (CT) scan three-dimensional reconstruction was compatible with an expansible lesion in the craniocervical junction. (c and d) CT scan was compatible with an intrinsic, expansible bone lesion, with multiple cysts, in the posterior aspect of the atlas. (e and f) CT scan showing the bone erosion of the posterior aspect of the atlas
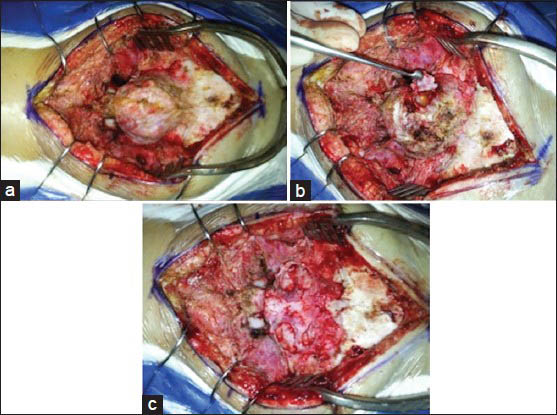
- Illustrative case − Patient 1-Intraoperative illustrations. (a) Aneurysmal bone cyst of the atlas. (b) Initial resection with the exposure of the arterial feeders. (c) Final aspect after total resection

- Illustrative case − Patient 2-A 28-year-old man presented at the emergency room with an important headache, somnolence, and nausea. Concomitantly, he complained of a mass growing in the back of his head for the last 6 months and reported having had a severe head injury 1 year prior to admission. (a and b) Computed tomography (CT) scan compatible with a posterior expansible lesion on the left side of his posterior fossa. (c and d) Magnetic resonance imaging T1-weighted with contrast compatible with an expansible bone tumor, with cystic walls, filled with blood vascularized. (e) Patient position. (f-h) Intraoperative illustrations with total resection (i and j) Postoperative CT scan showing total resection
The patient was discharged at the second day after surgery without complaints. No radiological evidence of recurrence after 9 months of follow-up.
Case 2
A 28-year-old man presented at the emergency room with an important headache, somnolence, and nausea, but with preserved muscular strength. Concomitantly, he complained of a mass growing in the back of his head for the last 6 months and reported a severe head injury 1 year prior to admission. A CT scan was compatible with an important hydrocephalus and a posterior expansible lesion on the left side of his posterior fossa [Figures 3a and b]. He underwent a ventriculoperitoneal shunt. MRI disclosed an expansible bone tumor, with cystic walls, filled with blood and extremely vascularized [Figure 3c and d]. A surgical resection was then performed with no prior embolization. Using a head holder, the patient was positioned prone and a midline incision with a transverse extension directed to the posterior aspect of the left mastoid was performed [Figure 2e]. The superficial skin and muscular layers were dissected from the bone, and the occipital artery was ligated at the beginning of the exposure, prior to directly entering the cyst. A subperiosteal dissection was then performed, exposing the tumor limits [Figure 3f and g]. The bony parts of the tumor were then resected piecemeal, drilling the residual intact bone edges until total tumor resection was evident [Figure 3h]. Once the contralateral occipitocervical junction was preserved, we opted for not fuse this patient with clinical and radiological follow-up of craniocervical instability.
The total surgical bleeding estimated was about 500 mL. The patient was discharged at the third day after surgery without neurological deficits. There was no recurrence after 7 months of follow-up [Figure 3i and j].
Discussion
ABC affects mainly patients between 10 and 20 years of age.[3] The incidence of craniocervical (occipito-C1-2) occurrence is not known. Up to 60-70% of the cases present with neurological deficits.[3] In our series, just patient two had neurological symptoms, secondary to his hydrocephalus. Many techniques are currently available for the treatment of ABCs, including curettage, partial resection, embolization of arterial feeders with polyvinyl alcohol particles, and radiation therapy.[45] Embolization was not used or considered because this modality of treatment was unavailable in our service. Albeit nonneoplastic in nature, complete removal of ABC can decrease recurrence and improve patient's quality of life. Subtotal resection is associated with early recurrence (up to 30% in the first year after surgery),[67] but total resection has been described as a highly morbid procedure because of important and even catastrophic bleeding.[35] Extensive resection of ABC in the spine may lead to instability, especially when the facet joints are involved, requiring a concomitant reconstructive procedure.[8]
In the craniocervical region, the specific literature of ABCs is sparse as well as its management, compared with ABC at other sites. We proposed a flow chart for diagnostic procedures and preoparative measures to treat ABC at the craniocervical junction [Figure 4]. Factors such as the close proximity to the cervicomedullary junction and the vertebral artery make the management more complex and challenging.[8] The relative low incidence of these lesions results in lack of expertise in choosing the best surgical option. In both our cases, total resection was possible with diligent and meticulous surgical technique and occlusion of their arterial feeders prior to directly approaching the tumors.
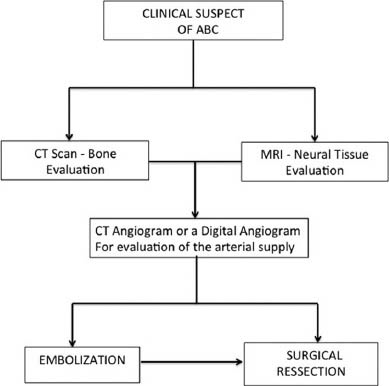
- A proposed flow chart for diagnostic procedures and preoperative measures required to treat craniocervical aneurysmal bone cysts
Conclusion
The surgical approach should be based on the knowledge of the anatomy of the region in order to achieve the occlusion of arterial feeders prior to surgical resection itself. In that regards, a vascular study such as a CT angiography or equivalent can be extremely useful for surgical planning.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Aneurysmal bone cyst of the spine. Report of 17 cases. J Neurosurg. 1985;63:685-90.
- [Google Scholar]
- Aneurysmal bone cyst of the mobile spine: Report on 41 cases. Spine (Phila Pa 1976). 2001;26:27-35.
- [Google Scholar]
- Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop B. 1998;7:286-92.
- [Google Scholar]
- Aneurysmal bone cyst. In: Musculoskeletal Tumor Surgery. Vol 2. New York: Churchill Livingstone; 1983. p. :1513-40.
- [Google Scholar]
- Aneurysmal bone cysts: Percutaneous embolization with an alcoholic solution of zein--series of 18 cases. Radiology. 1998;208:369-73.
- [Google Scholar]
- Aneurysmal bone cyst of the spine: Management and outcome. Spine (Phila Pa 1976). 1998;23:621-8.
- [Google Scholar]
- Aneurysmal bone cyst of spine: A review of literature. Arch Orthop Trauma Surg. 2008;128:1145-7.
- [Google Scholar]
- Aneurysmal bone cyst of the cranio-vertebral junction: Benign or malignant? J Neurosci Rural Pract. 2012;2:230-2.
- [Google Scholar]






