Translate this page into:
Role of erythrocyte sedimentation rate in ischemic stroke as an inflammatory marker of carotid atherosclerosis
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Inflammation mediates a key role in the pathogenesis of atherosclerosis which is an important cause of ischemic stroke. An elevated erythrocyte sedimentation rate (ESR) may, therefore, be a marker of the extent andor intensity of a general atherosclerotic process and thus a marker for advanced atherosclerosis heralding increased risk of arterial thrombosis leading to ischemic stroke.
Materials and Methods:
ESR was calculated in ischemic stroke patients by Westergren's method along with carotid sonography using high resolution 7.5 MHz techniques to find the prevalence of increased carotid intima-media thickness (CIMT) and presence of plaque according to Mannheim Carotid Intima-Media Thickness Consensus.
Results:
Average value of ESR in all patients was 27.89 ± 9.73 mm/h. A significant association was found between ESR and markers of carotid atherosclerosis, that is, high CIMT of more than 0.8 mm (P < 0.0001) and presence of plaque (P-0.026) in univariate analysis. Also, a significant positive correlation was found between ESR and serum fibrinogen, another inflammatory marker. (r = 0.88, P < 0.0001).
Conclusion:
The extent of inflammation may reflect in part the propensity of atherosclerotic lesions to lead to clinical disease. Study shows the association of ESR with markers of atherosclerosis confirming the strength of the inflammatory response associated with carotid atherosclerosis and might conceivably carry important prognostic information regarding occurrence of such catastrophic events in future.
Keywords
Arterial thrombosis
atherosclerosis
cerebrovascular disease
inflammation
ischemic stroke
plaque
Introduction
After coronary heart disease and cancer of all types, stroke is the third commonest cause of death worldwide. Stroke is a multi-factorial disease entity when looking at the etiology. Furthermore, the available knowledge about the patho-physiologic mechanisms of stroke is limited. This makes identification of factors causally related to the disease a challenge. Atherosclerosis through its complications is the leading cause of ischemic stroke. The natural history of atherosclerosis is a long process including a period during which “preclinical” involvement is detectable, although there are no clinical signs or symptoms of the disease. Therefore, the measurement of atherosclerosis itself from the beginning of its development represents an ultimate surrogate end-point of epidemiologic and therapeutic studies focused on atherosclerosis.
Inflammation mediates a key role in the pathogenesis of atherosclerosis.[12] Various cytokines, growth factors, and inflammatory cells are abundant in atheromatous plaques. The atherosclerotic vessel wall is the source of soluble adhesion molecules that mark inflammation.[34] Thus, the extent of inflammation may reflect in part the propensity of atherosclerotic lesions to lead to clinical disease.
During the past 15 years, modern high-resolution imaging ultrasound techniques have allowed researchers to quantify the presence and progression of early atherosclerosis in large peripheral arteries noninvasively in large population samples. Since the early study by Pignoli et al.,[5] demonstrating that the double-line arterial sonographic interface corresponds to intima-media thickness (IMT), the IMT of carotid arteries became the most studied preclinical parameter of vascular involvement. Recent technological advances in medical equipment have allowed noninvasive assessment of atherosclerosis in its early stages.[67] High-resolution B-mode ultra-sonography provides a noninvasive method for quantifying arterial wall thickening and it has been shown that carotid intima-media thickness (CIMT) is a strong predictor of cerebrovascular diseases.[6]
There are a large number of risk factors or predisposing factors known for atherosclerosis and probably many more are yet to be discovered. A number of markers or predictors of atherosclerosis have been developed and the search is still on to find out more reliable markers of atherosclerosis to increase the yield and cost-effectiveness of screening.
Erythrocyte sedimentation rate (ESR), which measures the tendency of red blood cells (RBCs) to aggregate, is a time-honored, routine analysis mainly used to screen for the presence of hidden inflammation, and to help in the diagnosis and follow-up of chronic diseases. The aim of the study was to evaluate the association of ESR with markers of carotid atherosclerosis in a cohort of ischemic stroke patients where the cause was assumed to be process of atherosclerosis.
Material and Methods
Study population
The study was conducted in patients admitted to the Medicine Department of King George's Medical University over a period of 1 year from August 2011 to August 2012. The study comprised of 92 ischemic stroke patients of more than 30 years of age with symptoms persisting for more than 24 h. Informed consent is taken from all the patients or their relatives before inclusion in this observational study.
Clinical evaluation of study population
Both imaging modalities and clinical assessment were used to select patients for this study. This included full-detailed clinical current and past history as well as imaging by magnetic resonance imaging to rule out other diseases of central nervous system mimicking ischemic stroke. This study was particularly for ischemic stroke patients where the cause was vascular atherosclerosis whether localized or generalized. To exclude other causes and to be specific for ischemic stroke of atherosclerotic origin following patients had been excluded from the study (1) Isolated transient ischemic attack; (2) Stroke as a result of apparent cardio-embolic origin; (3) Vasculitis syndromes causing stroke; (4) Subarachnoid haemorrhage, intracerebral hemorrhage; and (5) Patients with coagulation disorders (Antithrombin III, protein C, protein S deficiencies, and factor V Leiden and the prothrombin 20210A mutation). We also excluded patients with known inflammatory diseases, malignancies, and other hematologic diseases.
Biochemical parameters of study population
Study population was evaluated for different biochemical parameters and values compared with normal values. Patients were evaluated clinically and history was noted to know the prevalence of diabetes and hypertension in study group. Patients were considered as diabetic if fasting serum glucose was >126 mg/dL and random serum glucose was >200 mg/dL, whether they were a known case of diabetes mellitus or diagnosed during previous hospital admissions. Blood glucose was determined by the glucose oxidase method using Eco-Pak glucose reagent. Venous blood samples were taken after the subject had fasted for 12 h. The serum lipids (total cholesterol, high-density lipoprotein-cholesterol, and triglycerides) were analyzed by colorimetry (Bioclin). The low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald formula when triglyceride value was less than 400 mg/dL. Hemoglobin A1c was measured by ion exchange resin method using Erba kit. Other biochemical parameters were measured by kits available in our pathology.
Evaluation of ESR and other inflammatory markers
Measurement of the ESR was done using Westergren's technique within 12 h of stroke occurrence. ESR was first introduced by Westergren in 1921. The Westergren's method measures the rate of gravitational settling in 1 h of anticoagulated RBCs from a fixed point in a calibrated tube of a defined length and diameter held in an upright position. Erythrocytes normally have net negative charges and therefore repel each other. High molecular weight proteins that are positively charged, such as fibrinogen, increase in the acute-phase reaction, favoring rouleaux formation, thereby increasing the ESR. Plasma viscosity correlates with ESR. The normal value of ESR for male is 0 - 15 mm/h and for females is 0 - 20 mm/h. In this study, we have overlooked the disparities in values of ESR for gender differences. This was done for not complicating the statistical data analysis.
Fibrinogen is a high molecular weight glycoprotein, which plays a major role in hemostsis and an important determinant of vascular atherogenicity. Levels of fibrinogen from 2.33 to 4.96 g/L are taken as normal. Levels higher then this indicate an increased atherogenicity of the blood.
Carotid ultrasound
CIMT is a noninvasive alternative marker of atherosclerotic disease which is a relatively easy, noninvasive technique to identify atherosclerosis. The patient kept supine with slight hyperextension and rotation of the neck in the direction opposite the probe. A linear array transducer with a multiple frequency (7 - 12 MHz) attached to a high-resolution B mode ultrasound system was used to acquire images by a single-sonographer blind to clinical data of subjects (by Toshiba-Xario sonography instrument). Manual measurement of IMT was performed in the common carotid artery, at both sides, in a region free of plaque located approximately 20 mm from bulb. At least three values were obtained in different sites of this segment and the mean value of six measurements (three from each side) was used for analysis. Further common carotid and internal carotid arteries were screened for any plaque and its characteristics. The protocol we followed was according to Mannheim Carotid Intima-Media Thickness Consensus (2004-2006).[8] The consensus recommends the following definitions for ultrasound characterization of IMT and atherosclerotic plaque:
-
CIMT is a double-line pattern visualized by echotomography on both walls of the common carotid arteries in a longitudinal image. It is formed by two parallel lines, which consist of the leading edges of two anatomical boundaries: The lumen-intima and media-adventitia interfaces
-
Plaque is a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding CIMT value or demonstrates a thickness 1.5 mm as measured from the media-adventitia interface to the intima lumen interface [Figure 1].
 Figure 1
Figure 1- Carotid sonography showing increased carotid intima-media thickness and carotid plaque
While CIMT describes early changes, carotid plaque specifies more advanced stage of atherosclerosis. The cut off value for normal CIMT in our study was taken as 0.8 mm as in different studies this value of CIMT was found to correlate well with vascular risks as seen by Hansa et al.,[9] It was also found to appropriately predict risk among Indians.[10]
Statistical analysis
The results were presented in mean ± standard deviation and percentage. Chi-square test was used to compare the dichotomous/categorical variables. The unpaired t-test used to compare two means. The P < 0.05 was considered as significant. All the analysis was carried out by using SPSS (Statistical Program for social sciences) 15.0 versions.
Results
Characteristics of the study subjects
The study was conducted over 104 cases out of which 12 were withdrawn due to various reasons (including death and leaving against medical advice); hence, only 92 were finally included in the study. About one-third of the patients were >70 years (33.7%) and between age group 61 and 70 years (32.6%). A total of 25% of the patients were between 50 and 60 years and 8.7% were <50 years. More than half (60.9%) of the patients were males. In our study, hemiparesis was seen in 88% of the patients and cranial nerves involvement in 18.5%. Aphasia was present in 26.1% patients. Of all the patients of ischemic stroke in our study, previous history of hypertension was present in 22.8% and diabetes in 32.6%. More than one-third (38%) were tobacco chewers, 21.7% were smokers, and about 20.7% were alcoholic.
The average value of ESR in all patients was 27.89 ± 9.73 mm/h in this cohort. The mean fasting blood glucose of all ischemic stroke patients was 126.26 ± 47.43 mg/dL and postprandial value was 205.8 ± 76.94 mg/dL. Both these values were much higher than of average Indian population even more than diabetic limit.
The average values of the risk factor indicator parameters in our study were greater in stroke patients as compared with the normal population, like serum fibrinogen, homocystiene, triglycerides, total cholesterol, LDL, and very LDL cholesterol were found to be raised Table 1.

Carotid sonographic parameters
Carotid ultrasound shows increased value of average mean CIMT in this cohort of ischemic stroke patients. The average value of mean CIMT in all stroke patients was 0.92 ± 0.2844 mm and a high CIMT was observed in 73.9% (The cut off value for normal CIMT for Indian population in our study was taken as 0.8 mm as in different studies this value of CIMT was found to correlate well with vascular risks). The prevalence of carotid plaque was 40.2% in our study, with Type 2 echogenicity was the most common type, as was seen in 40.5% patients; while Type-3 in 37.8% of them. The plaque surface was irregular in 45.9% of the patients enrolled.
ESR as a marker of chronic inflammation
A significant positive correlation was found between ESR and serum fibrinogen another inflammatory marker (r = 0.81, P < 0.0001) [Figure 2].
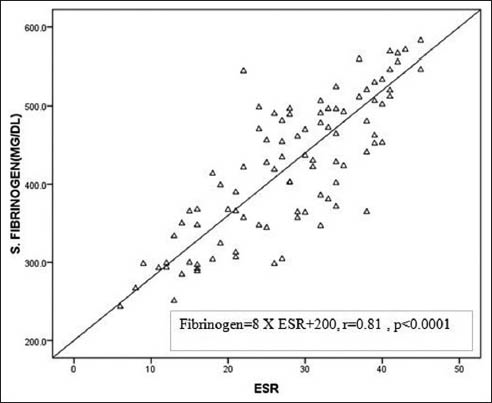
- Correlation of erythrocyte sedimentation rate with serum fibrinogen
Association of ESR with markers of atherosclerosis (CIMT and carotid plaque)
A significant association was found between laboratory marker of systemic inflammation, that is, ESR and the carotid artery IMT (P < 0.001) and similarly ESR was found significantly associated with carotid plaque (P = 0.026), both of which are noninvasive measures of atherosclerosis [Table 2, Figure 3].


- Association of erythrocyte sedimentation rate with carotid intima-media thickness and carotid plaque
Discussion
Atherosclerosis is basically chronic inflammation in the arterial wall[2] and this has prompted a search for inflammatory markers as indicators of coronary heart disease and cerebrovascular disease risk, and, for example, fibrinogen and C-reactive protein have gained some popularity in this regard.[111213]
In 1988, the International Committee for Standardization in Haematology suggested that the ESR might be a particularly robust and appropriate test for monitoring chronic inflammatory processes since it is sensitive to both fibrinogen and immunoglobulins.[14] Accordingly, a fast and inexpensive ESR test might be a valuable tool for assessing the strength of the inflammatory response associated with atherosclerosis and might conceivably carry important short- and long-term prognostic information.
Another possibility suggested by many authors is that rheological characteristics of blood contribute to the pathogenesis of cerebrovascular diseases. The major components of blood viscosity are the blood cell mass (i.e., the hematocrit), the intrinsic resistance of the plasma to flow (commonly measured by capillary viscometry), and RBC aggregability, which can be estimated through the ESR but definite proof of this hypothesis is still lacking.
Accelerated erythrocyte aggregation is caused by large, asymmetrical plasma proteins inhibiting the negative electrical forces which normally keep the erythrocytes apart. The proteins known to be mainly responsible for this effect are fibrinogen, immunoglobulins, lipoproteins, and alpha-2 macroglobulin.[15] Some of these are independent risk factors in causation of ischemic stroke shown by few studies.[16] Our study also shows significant correlation between serum fibrinogen and ESR suggesting this interdependence. How various cytokines, chemokines, and other substances liberated from atheromatous tissue[2] influence the ESR is largely unknown, but proteins which facilitate thrombocyte aggregation and cellular adhesion and migration might conceivably also alter erythrocyte membranes and increase their propensity to aggregate. The suggestion that the ESR increment occurs in response to stroke is also strengthened by the study of Emsley et al.,[17] who have shown an increase in ESR values in stroke patients in comparison with those in nonstroke patients with atherosclerosis, the most common pathological condition in ischemic stroke. This study has strengthened the view that atherosclerosis which is a quite common cause of ischemic stroke can be assessed by ESR as association of ESR was significant with CIMT and carotid plaque.
Since ESR is a nonspecific marker of inflammation and is affected by other factors, the results must be used along with the other clinical findings. Since the stroke patients display multiple risk factors for atherosclerosis such as hypertension, diabetes mellitus, and smoking, it is possible that they presented a preexisting proinflammatory/procoagulant condition, which may, at least in part, contribute to the increase in ESR values soon after the stroke.
However, there are some important limitations in our study. The patients assessed were from a potentially biased population that may have various undiagnosed ailments not uncovered while selection leading to elevated ESR values. The cause for the stroke in all patients might not be atherosclerosis and other causes could have affected the results of study. Also, the data did not compare these findings and values with normal healthy population individuals as prevalence of anemia is quite high in India and large number of apparently healthy will have high ESR.
Conclusion
In the present study, we analyzed the relationship of inflammatory biomarker-like ESR, to the degree of carotid atherosclerosis, as a surrogate marker for the atherosclerotic disease predicted by another marker, that is, carotid artery intima media thickness and carotid plaque and found both significantly associated. Study illustrates the complex interaction between the inflammation and coagulation systems that is of great importance in thrombogenesis, leading to catastrophic events like ischemic stroke.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131-8.
- [Google Scholar]
- Soluble vascular cell adhesion molecule-1 as a biohumoral correlate of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2646-54.
- [Google Scholar]
- Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1997;96:4219-25.
- [Google Scholar]
- Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation. 1986;74:1399-406.
- [Google Scholar]
- Intima media thickness as a surrogate marker for generalized athrosclerosis. Cardiovasc Drugs Ther. 2002;16:341-51.
- [Google Scholar]
- Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75-80.
- [Google Scholar]
- Carotid intima-media thickness and coronary artery disease: An Indian perspective. Asian Cardiovascular Thorac Ann. 2003;11:217-21.
- [Google Scholar]
- Carotid intima-media thickness as an independent predictor of coronary artery disease. Indian Heart J. 2001;53:458-62.
- [Google Scholar]
- Haemostatic function and ishaemic heart disease: Principal results from the Northwick Park Heart Study. Lancet. 1986;2:533-7.
- [Google Scholar]
- Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973-9.
- [Google Scholar]
- Association of inflammatory markers with intima-media thickness and acute ischemic stroke. J Neurol Sci. 2009;26:153-64.
- [Google Scholar]
- Guidelines on selection of laboratory tests for monitoring the acute phase response. International Committee for Standardization in Hematology (expert panel on blood rheology) J Clin Pathol. 1988;41:1203-12.
- [Google Scholar]
- Clinical Chemistry in Practical Medicine. (5th ed). Lund: Student Literature; 1986.
- [Google Scholar]
- Carotid intima-media thickness and apolipoproteins in patients of ischemic stroke in a rural hospital setting in central India: A cross-sectional study. J Neurosci Rural Pract. 2012;3:21-7.
- [Google Scholar]
- An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93-101.
- [Google Scholar]






