Translate this page into:
Endovascular neurointervention success and complication rates in the first year of independent practice in a suburban hospital setup
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Endovascular neurointervention (interventional neuroradiology) is a highly demanding science requiring deep understanding of disease, anatomy, clinical skills and manual dexterity, consequently with a long learning curve and thus posing significant challenges to a physician entering new into the competitive arena.
Aim:
To evaluate the procedural success, complications and outcome in the first year of independent endovascular neurointervention practice in a suburban hospital.
Materials and Methods:
Retrospective analysis of prospectively maintained data of all diagnostic and therapeutic neurointerventional cases performed by the author between the period of January 02, 2012 and December 31, 2012.
Results:
A total of 61 procedures were performed. The performance success rate of the diagnostic procedures was 100% (38/38) and that of therapeutic procedures was 82.6% (19/23). The periprocedural complication rates were nil and 13%, respectively, for diagnostic and therapeutic procedures. The 3-month patient outcome for therapeutic procedures was good outcome (Modified Rankin Scale <2) in 87% cases (20/23), and poor outcome in 13% (2 dead and 1 debilitated with Modified Rankin Scale of 3).
Conclusion:
For a well-trained endovascular neurointerventionalist, the first year of practice had high procedural success rate and acceptable complication with patient outcome rates comparable to the existing literature.
Keywords
Acute stroke
aneurysm coiling
cerebral angiography
combined lysis of thrombus in brain ischemia using transcranial ultrasound and systemic tissue plasminogen activator
sonothrombolysis
embolization
endovascular neurointervention
Introduction
Endovascular neurointerventions form a significant part of a neuroradiologist's practice, and can be very challenging especially at the start of the individual practice. It also demands a very strong coordination between multiple departments and individuals. The author had undergone 3 years of training program followed by 1 year and 4 months of supervised practice before starting an independent practice within a team also having vascular neurophysician and neurosurgeon, with a dedicated stroke care unit and a neuro-rehabilitation team. The challenges at the start of the practice are many, and the author aimed to evaluate the procedural data in the first year of practice. This would set the tone for the further practice patterns of the author as well as serve as a guide to other young neurointerventionalists.
Materials and Methods
A registry of all cases between January 02, 2012 and December 31, 2012 performed independently by the author was maintained. The data was evaluated retrospectively after 3 months of the last case. The author had at his disposal a dedicated interventional cathlab suite with flat panel (Siemens Artis Zee, Erlangen, Germany) machine, along with trained technicians and nurses (predominantly with cardiac background and short term trainings of neuro- and/or peripheral interventions). All cases were also clinically evaluated by one or both of a vascular neurophysician and neurosurgeon. Most of the cases were managed in a dedicated stroke care unit. Whenever required, the patients were managed in surgical intensive care unit, ward, or daycare units. A dedicated physiotherapy and neurorehabilitation team took care of the patients whenever needed.
The author accepted these broad categories of patients: Diagnostic digital subtraction angiography (DSA) of adults, with any kind of cerebro- or spinal vascular pathology; interventions for acute ischemic stroke, aneurysms, tumor embolization, and extracranial vascular malformations. The author performed two types of interventions for acute stroke: either CLOTBUST sonothrombolysis or intraarterial thrombolysis (IAT) depending upon the clinical profile and the imaging features, in discussion with the neurophysician.
The following broad groups were not accepted by the author: Pediatric age group patients, intracranial arteriovenous malformation (AVM)/dural arteriovenous fistula (DAVF) embolization, extracranial or intracranial angioplasty with or without stenting (except in the setting of acute stroke); stent-assisted aneurysm coiling (balloon-assisted aneurysm coiling was accepted).
Observations
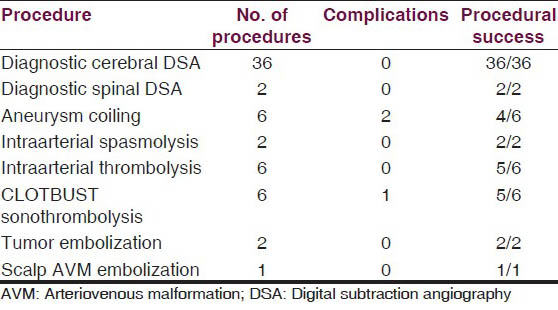
The type of procedures performed with individual numbers are given below [Table 1].
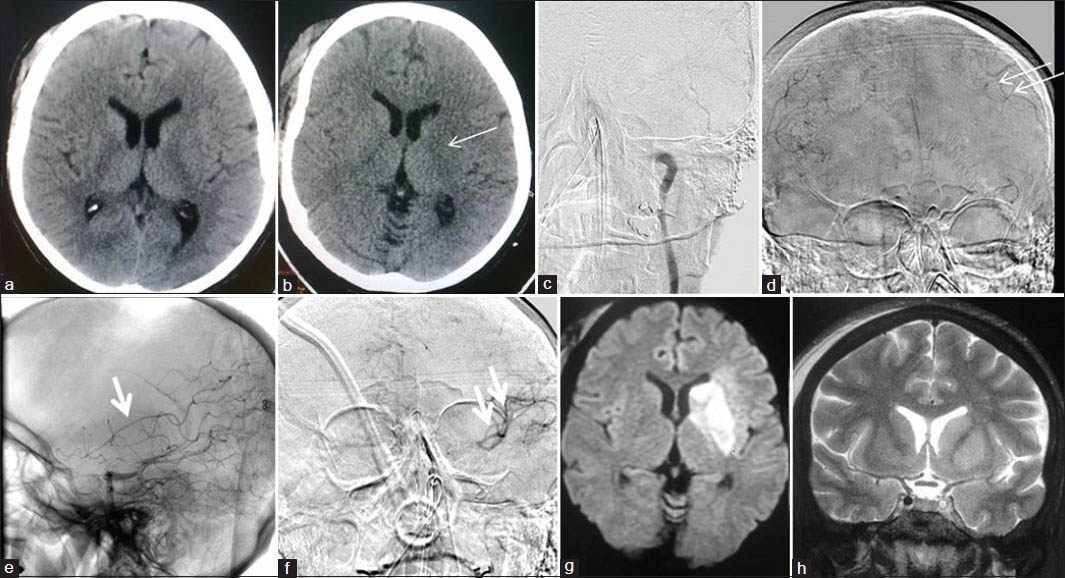
There were three direct procedure-related complications: One case of large vessel thromboembolism, and another case of distal vessel thromboembolism [Figure 1], both during aneurysm coilings. One case of acute stroke developed oral bleeding during CLOTBUST sonothrombolysis.
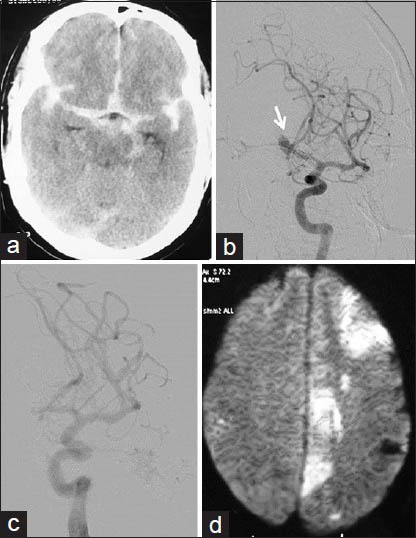
- Thromboembolic complication of aneurysm coiling: A 54-year-old male with grade 1 SAH and ruptured Acom artery aneurysm; endovascular aneurysm coiling done with complete aneurysm exclusion from circulation with distal thromboemboli. (a) NCCT head at admission showing diffuse SAH. (b) Left ICA angiogram showing Acom aneurysm directed superiorly, and to right side (arrow). (c) Postcoiling left ICA angiogram showing well coiled aneurysm completely excluded from circulation. (d) Next morning DWI image showing restricted diffusion areas in left MCA and ACA territories
All but four procedures could be completed satisfactorily with all information obtained during diagnostic procedures and the intended therapy provided in interventional procedures. One case of aneurysm coiling could only be done partially [Figure 2] and another case could not be done due to large thromboembolism and patient deteriorating on table. A case of acute stroke could be only partially recanalized during thrombolysis and another case of CLOTBUST sonothrombolysis had to be abandoned due to oral bleeding during the therapy.
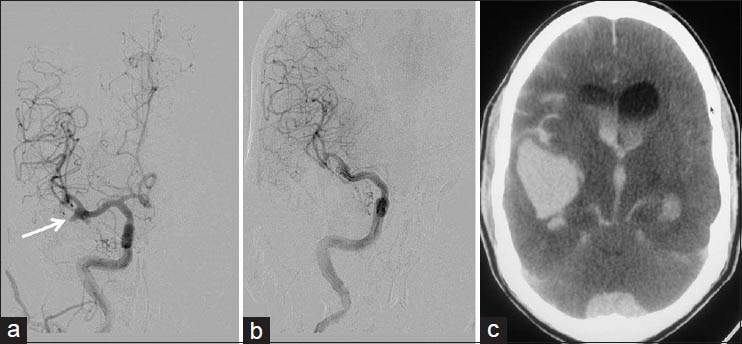
- Rerupture of partially coiled aneurysm: A 46-year-old male with Grade 1 SAH and ruptured right MCA bifurcation aneurysm; after an hour of extubation after partial coiling, patient developed left hemiparesis followed by pupillary dilatation. (a) Right ICA injection frontal view showing MCA bifurcation aneurysm with broad base (arrow). (b) Postcoiling right ICA injection frontal view showing secured dome of the aneurysm with the patent lower part (c) CT done after patient developed hemiparesis, showing increased SAH with hydrocephalus, and large right Sylvian/temporal hematoma
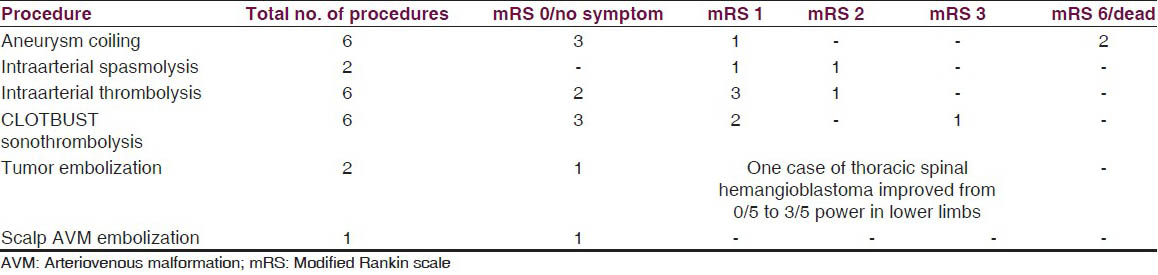
Three-month outcome data of the patient's interventions are summarized in Table 2.
Discussion
Endovascular neurointervention is an interdisciplinary modality of patient management offering minimally invasive diagnostic and therapeutic options to often challenging and difficult neurovascular cases.[12] In this regard, the start of individual career of any neurointerventionalist is particularly difficult and fraught with risk of complications and poor outcomes.
However, adequate training and supervision combined with inherent ability to select appropriate patients and diseases can produce good results comparable to those in the literature, and give encouragement to the practitioner to progressively embark on a journey of learning and teaching.[23456]
The author had undergone 3 years training program followed by 1 year and 4 months of supervised practice in a large group of neurointerventionalists, before starting fully independent practice (in a 330-bed suburban hospital without any existent neurointerventional services). This allowed understanding the nitty-gritty of choosing the right case authoritatively as also to formulate management plans or refer out the patients.
The author refused cases that demanded extreme understanding of the anatomy, pathology, and physiology or needed fantastic dexterity.
Such cases included complex AVMs and DAVFs, intracranial angioplasty and stenting, neck vessel angioplasties in patinets with comorbidities, severe flow restricting stenosis or poor vessel/plaque morphology.
The authors mostly concentrated on providing complete diagnostic services and therapy for acute cases to the hospital and included acute stroke and aneurysms.
Diagnostic services
Diagnostic angiographies were the largest group of procedures. Altogether, 36 cases of diagnostic cerebral and 2 spinal DSAs were performed. Complete information could be extracted in all cases and therapeutic planning could be done. All types of cases were taken up and included aneurysms, AVM, DAVF, extracranial or intracranial stenotic conditions, and tumors. No complications, related to the puncture site or neurological, were observed. This compares well with the literature,[78910] for example, a large series of 19,826 cases by Kaufmann et al.[7] had shown 4.2% puncture site complications, 2.63% neurologic deficits (including, 0.14% stroke with permanent disability), and 0.06% death. In yet another series of 2899 cases by Willinsky et al.,[8] there were 1.3% neurologic complications, including 0.5% permanent disability from stroke.
Various factors have been reported to be associated with complications of angiography,[78910] and include increasing age, atherosclerotic cerebrovascular disease, subarachnoid hemorrhage (SAH), frequent transient ischemic attack, cardiovascular disease, and long fluoroscopic times.
In the elective patient management practice, the author makes sure that noninvasive angiography (computed tomography angiography [CTA] and or magnetic resonance angiography [MRA]) is performed in such patient groups as far as possible, as also in patients with long-term diabetes mellitus, hypertension, renal disease, severe obesity (to avoid groin puncture site complications).
Acute stroke
The author is a neuroradiologist, involved in reading the patient's imaging as well as doing Transcranial Doppler (TCD) and performing the endovascular procedures. After deciding upon the necessity of an intervention, in collaboration with the vascular neurophysician, the treatment options were discussed with the patient and/or the family. All the three modes of interventions could be offered – intravenous thrombolysis (IVT), CLOTBUST sonothrombolysis or IAT. Depending upon the individual case (predominantly the presence of large vessel occlusion) and patient/family preference, one of the three was done. The author was entrusted upon with the latter two types of intervention.
In all, six cases of CLOTBUST sonothrombolysis [Figure 3] and six cases of IAT were done. Five more cases were taken up for IAT, but after the angiogram, intervention was deemed not suitable/not required and hence not proceeded with [Figure 4]. These five cases are included under the head of ‘diagnostic cerebral angiography’.
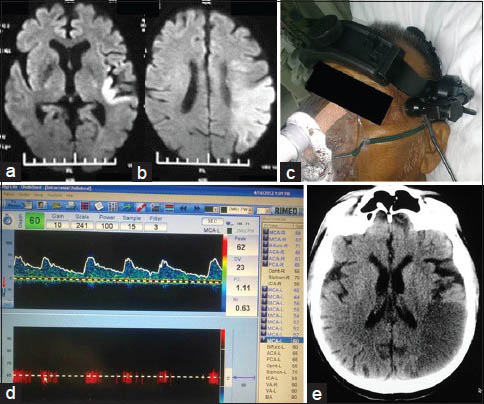
- CLOTBUST sonothrombolysis therapy with good outcome: A 70-year-old male with CAD, CKD and acute left MCA territory stroke of 4 h. (a) Admission DWI at level of basal ganglia. (b) DWI at level of centrum semiovale; diffusion restriction noted in left temporo-parietal region with DWI - ASPECTS of 6. (c) TCD frame fixed onto the patient's head. (d) TCD waveform and parameters, at the end of the procedure; flow is still not very good but the velocities are within normal limits. (e) NCCT head done next morning with established infarct in left temporoparietal area
- A 43-year-old female with right hemiparesis since 2 h (a) Admission CT head -ASPECTS 10; IVT was started. (b) Post-IVT CT with ischemia in left gangliocapsular region (thin arrow). (c) Left CCA injection, showing complete occlusion of left petrous ICA. (d) Right CCA injection, AP view. (e) Left VA injection, lateral view, and 4. (f) Left VA injection, AP view, show good collaterals; no further intervention done. (g) DWI next morning, showing restriction in left gangliocapsular area. (h) Coronal T2WI showing patent left ACA, MCA and supraclinoid ICA with still occluded cavernous ICA
The technique of CLOTBUST sonothromobolysis used was as described in literature.[11] The results were excellent and well above the published results. We went through the reasons for the success and found the following:
-
Well-oiled acute stroke management machinery was in place. Our team tried to cut down the time of transit between various rooms and did not opt for prolonged imaging protocols[12] or sedation. The average time for patient to move through the emergency to computed tomography (CT) and then to stroke care unit was 20 min and in no case was more than 30 min
-
The TCD procedure was started very early, as soon as the patient returned from the CT room into the stroke care unit. In fact the rtPA (recombinant tissue plasminogen activator) injection could be started at an average time of approximately 30 min after the start of the TCD
-
A trained nurse stood by the side of the patient making sure that the TCD went on smoothly, and also did the mandatory neuro-vital monitoring
-
The author himself made sure that the CT interpretation was done on the console
-
The patient and attendants were counseled personally during the transit
-
All cases had start of rtPA injection within 1 h of arrival
-
The TCD continued for a full duration of 2 h, irrespective of the change in neurological functions and the status of the rtPA injection.
For IAT, the author mostly used rtPA and mechanical thrombectomy was done in one of the cases, along with 2 mg rtPA infusion. Out of the six cases of IAT, TICI 2b/TICI 3 (thrombolysis in cerebral infarction; TICI[13]) reperfusion was achieved in five, and all these cases had good outcome at 3 months. One case had minimal recanalization and fared average at 3 months with mRS of 2. All combined, the results of IAT compare with those in existing literature.[1415]
Aneurysm and/or subarachnoid hemorrhage management
In the integrated stroke care services offered in the institute, the author was involved at each step of patient care of in-patients with aneurysms (ruptured or unruptured). All CT scans were read by the author, and daily TCDs on SAH patients were done.
Patients/attendants were offered either CTA or DSA for SAH evaluation and depending upon their choice, one was done.
Aneurym coiling[16] was performed by the author after discussion with the stroke team. In all, six coiling procedures were done. One patient had thromboembolic event, which could not be recanalized in spite of all techniques tried, and another patient with right middle cerebral artery (MCA) bifurcation aneurysm died after 4 h of a partial coiling procedure due to massive rebleed [Figure 2].
In the remaining patients, complete or near complete aneurysm filling could be achieved, with only interstitial filling or dog-ear remnants seen after the completion of procedure. Balloon assistance[17] was needed in two cases, and no thromboembolic complications were seen in either.
Tumor embolization
Two cases of tumor embolizations were done. One was a case of a spinal hemangioblastoma, which had undergone surgery twice earlier, no doubt failed, and taken up for embolization. The tumor could be partially embolized and the patient then underwent a successful repeat surgery. Another patient has a large sacral Schwannoma, which was embolized near completely via both internal iliac arterial branches, and then underwent successful tumor removal [Figure 5].
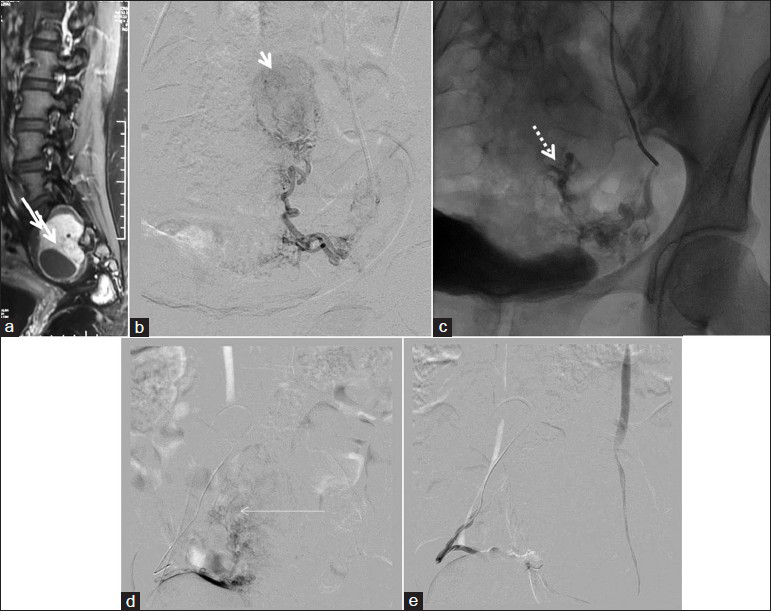
- A 39-year-old female with sacral Schwannoma; PVA particle embolization with complete tumor devascularization (a) Sagittal postcontrast T1WI shows enhancing dumbbell shaped sacral mass. (b) Left internal iliac artery angiogram showing the oval moderately vascular mass. (c) Postembolization, fluoroscopic spot view showing contrast stagnation. (d) Right internal iliac artery injection, showing the enlarged tortuous feeders and the moderately vascular mass. (e) Post-PVA particle embolization, right internal iliac artery injection, showing complete cessation of flow to the tumor
AVM management
The author performed diagnostic angiographies of AVMs, ruptured or unruptured. The reports were made as per Valavanis et al.[18] Discussions were done with the neurophysician and neurosurgeon and further planning done. No embolizations were done (the author has now started embolizing AVMs in the 2nd year of practice).
The role of adequate and appropriate training
The author strongly feels that a neurointerventionalist has to undergo a proper training program with adequate number of cases, academic sessions, lectures and discussions, and most important of all, adequate duration.[123456] This has been substantiated in a number of articles recently, with factors independently associated with a decreased risk of neurologic complication being increasing chronologic year in which the procedure was performed and involvement of a trainee in the procedure. Also, now more and more low volume centers have started taking up neurointerventional cases.[1920] Background knowledge of diagnostic tools, clinical knowledge and acumen and dedication is must. Shortcut ‘observerships’ or ‘fellowships’ are no way to take the field forward. Many authors today feel that neurointerventional training programs should be abandoned altogether. While this is not at all mandatory, the programs have to be made sufficiently ‘lengthy’ and ‘proper’ with focus on the ‘basics’ to ensure that only dedicated and interested physicians enter the field. This can ensure appropriate patient management and healthy evolution of the field.
Conclusion
The author setup an interventional neuroradiology service in a suburban hospital, completing a dedicated stroke care team, and the first year of the service produced high procedural success rate and acceptable complications with patient outcome rates comparable to the existing literature.
Acknowledgments
Dr. Ishwar C Premsagar, Head of Neurosurgery, Sri Balaji Action Medical Institute, Paschim Vihar, New Delhi Dr. Rajul Agarwal, Head of Neurology, Sri Balaji Action Medical Institute, Paschim Vihar, New Delhi Dr. Sandhya Koche, Consultant Neurology, Sri Balaji Action Medical Institute, Paschim Vihar, New Delhi Dr. BS Sodhi, Senior resident, Sri Balaji Action Medical Institute, Paschim Vihar, New Delhi.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Training, competency, and credentialing standards for diagnostic cervicocerebral angiography, carotid stenting, and cerebrovascular intervention: A joint statement from the American Academy of Neurology, the American Association of Neurological Surgeons, the American Society of Interventional and Therapeutic Neuroradiology, the American Society of Neuroradiology, the Congress of Neurological Surgeons, the AANS/CNS Cerebrovascular Section, and the Society of Interventional Radiology. J Vasc Interv Radiol. 2004;15:1347-56.
- [Google Scholar]
- Qualification requirements for performing neurointerventional procedures: A report of the practice guidelines committee of the American Society of Neuroimaging and the Society of Vascular and Interventional Neurology. J Neuroimaging. 2008;18:433-47.
- [Google Scholar]
- The learning curve for neuroendovascular procedures: How important is it? Neurology. 2009;72:1974-5.
- [Google Scholar]
- Simulation for Neurointervention. Simulators serve as a valuable training aid for new neurointerventional fellows and others who may be inexperienced with catheter-endovascular techniques. Endovascular Today. 2008;11:71-4.
- [Google Scholar]
- Training with simulation improves residents’ endovascular procedure skills. J Vasc Surg. 2007;45:149-54.
- [Google Scholar]
- Learning curve of Wingspan stenting for intracranial atherosclerosis: Single-center experience of 95 consecutive patients. J Neurointervent Surg 2013
- [Google Scholar]
- Complications of diagnostic cerebral angiography: Evaluation of 19,826 consecutive patients. Radiology. 2007;243:812-9.
- [Google Scholar]
- Neurologic complications of cerebral angiography: Prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522-8.
- [Google Scholar]
- Low rate of complications of cerebral angiography in routine clinical practice. Neurology. 2001;57:2012-4.
- [Google Scholar]
- Complications of modern diagnostic cerebral angiography in an academic medical center. J Vasc Interv Radiol. 2009;20:442-7.
- [Google Scholar]
- Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170-8.
- [Google Scholar]
- Multimodal imaging for acute stroke: When is it worth it? Neurology. 2013;81:608-9.
- [Google Scholar]
- Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109-37.
- [Google Scholar]
- Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-11.
- [Google Scholar]
- Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke. 1999;30:2598-605.
- [Google Scholar]
- Procedural complications of coiling of ruptured intracranial aneurysms: Incidence and risk factors in a consecutive series of 681 patients. AJNR Am J Neuroradiol. 2006;27:1498-501.
- [Google Scholar]
- Balloon-assisted coil embolization of intracranial aneurysms: Incidence, complications, and angiography results. J Neurosurg. 2006;105:396-9.
- [Google Scholar]
- Endovascular treatment of cerebral arteriovenous malformations with emphasis on the curative role of embolisation. Interv Neuroradiol. 2005;11(Suppl 1):37-43.
- [Google Scholar]
- Cerebral aneurysm treatment is beginning to shift to low volume centers. J Neuro Intervent Surg 2013 Epub ahead of print
- [Google Scholar]
- Neurointerventional procedural volume per hospital in United States: Implications for comprehensive stroke center designation. Stroke. 2012;43:1309-14.
- [Google Scholar]






