Translate this page into:
Miliary brain metastases from occult lung adenocarcinoma: Radiologic and histopathologic confirmation
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
“Miliary brain metastases”, also termed as “Carcinomatous encephalitis”, are an extremely rare form of cerebral metastasis. Here in this article, we report a 52 year-old male patient with miliary brain metastases originating from occult lung adenocarcinoma. There were no significant findings on his initial physical and neurological examinations except limited cooperation. Brain computed tomography revealed edematous regions at the inferior sections of both parietal lobes. Then after, the contrast-enhanced magnetic resonance imaging revealed innumerable multi-dimensional lesions associated with surrounding edema on T2-weighted images. The proton magnetic resonance spectroscopy revealed increases in the choline and lipid peaks with decreased N-acetylaspartate in a similar manner with metastatic brain tumors. Histopathological findings pointed out that malignant epithelial tumor metastasis were originating in primary lung adenocarcinoma. Despite the advances in technical equipments and medical knowledge, miliary metastatic brain tumors are quite rare and the differential diagnosis is difficult. Our aim in this article was to present this rare case in which the lung was thought to be the primary focus; and outline the radiological characteristics. Also, we believe that the findings presented by proton magnetic resonance spectroscopy may contribute to making a differential diagnosis.
Keywords
Miliary brain metastases
computed tomography
magnetic resonance imaging
magnetic resonance spectroscopy
cerebral biopsy
Introduction
“Carcinomatous encephalitis”, also termed as “miliary brain metastases”, is an extremely rare form of brain metastasis and only 5% of all metastatic brain tumors consist of five or more lesions. Clinical findings are very similar to metabolic/toxic or infectious encephalopathy on initial neurologic examination of the patients with miliary brain metastases. Therefore, the physicians usually pursue other more frequent diseases that could explain clinical manifestations of these patients.[12]
In addition to a metastatic brain tumor, radiological differential diagnosis of these lesions includes miliary brain tuberculosis, neurocysticercosis or toxoplasmosis. Nevertheless, because of similarity in appearances on different conventional magnetic imaging (MRI) sequences, (MRI) cannot always provide enough information for differentiation of these lesions.[3] Although due to the different metabolic features of neoplastic and non-neoplastic lesions, the exact diagnosis can only be made by cerebral biopsy or by proton magnetic resonance spectroscopy (MRS) methods to enable differentiation between these lesions. The elevated choline (Cho) levels along with spectrophotometric data analyses of other cerebral metabolite concentrations (Cho/Cr and NAA/Cr ratios) are the main markers for differentiation between neoplastic and non-neoplastic lesions by proton MRS.[4]
Even if proton MRS does not change the leading diagnosis, it may rule out differential diagnosis and thereby it may reduce the diagnosing interval in patients with contrast enhanced miliary brain lesions on conventional MRI. Thus, early diagnosis and treatment can prevent morbidity, and can reduce the mortality rates in such patients.[4]
Case Report
A 52-year-old previously healthy male patient was admitted to our emergency department with complaint of seizure and loss of consciousness after a minor head trauma. On admission, his physical and neurological examinations were normal except limited cooperation. There was no significant feature in his past medical history.
Laboratory investigations including blood biochemistry, whole blood count, erythrocyte sedimentation rate, thyroid functions, and urinalysis results were all within normal limits. Electroencephalogram showed diffuse slowing pattern in both cerebral hemispheres.
In radiological examination, the plain skull films were normal. Brain computed tomography (CT) revealed edematous regions at the inferior sections of both parietal lobes [Figure 1]. Afterwards, contrast-enhanced MRI revealed innumerable multi-dimensional lesions on both supra- and infratentorial areas. Majority of these lesions were oval in appearance, with iso- to low-intensity signals on T1- and high-intensity signals on T2-weighted images associated with surrounding edema. Most of them had homogenous and others had ring-like enhancement of contrast medium. In the same session MRS was performed; because of an increase in the Cho level (1.6966) with elevated Cho/N-acetylaspartate (NAA) (1.6966/1.4029=1.2093) and lowered NAA/Creatine (Cr) (1.4029/1.0950=1.2811) ratios on proton MRS, these miliary lesions were thought to be consistent with metastasis. Presence of moderate lipid peaks was thought to be related to tissue necrosis as seen in high grade malignancy [Figures 2a–d]. Although, in addition to metastatic brain tumor, infectious pathologies such as miliary brain tuberculosis, neurocysticercosis and toxoplasmosis were also included in differential diagnosis because of the similar radiological appearances. Interestingly, whole-body PET CT revealed no primary tumoral focus, and tumor markers including NSE, CEA, CA 19-9 and serum S100β protein were within the normal range. Lumbar puncture revealed normal opening pressure, and CSF analysis showed only 1 cell/μl with normal protein (0.32 g/L), albumin (0.3 g/L), lactate (1.8 mmol/L) and glucose (0.72 g/L) levels. Cytopsin analysis of CSF did not reveal any abnormality. Multiple CSF cultures were also negative. Additionally, serological and immunological examinations of the blood and CSF revealed no evidence of parasitic disease. All other analyses, including HIV serology, anti-Hu 1 antibody and tuberculin skin test were negative. In order to reveal the nature of brain lesions, a cerebral biopsy was performed with stereotactic guidance and the following pathologic examination revealed malignant epithelial tumor infiltration characterized by solid nests of atypical cells having pleomorphic nuclei and eosinophilic cytoplasm [Figures 3a and b]. Immunohistochemistry was performed on tissue sections with use of a Ventana Automated Immunostainer. The antibodies used included: Cytokeratin 7 (OV-TL12/30, 1:150, Neomarkers), cytokeratin 20 (Ks20.8, 1:100, Neomarkers), TTF-1 (8G7G3/1, 1:50, Neomarkers), and Surfactant (32E12, 1:400, Novocastra). Immunohistochemically, neoplastic cells were positive for cytokeratin 7 and TTF-1 whereas cytokeratin 20 and Surfactant were negative [Figures 3c and d]. In the light of histopathological findings, these lesions thought to be moderately or poorly differentiated lung adenocarcinoma metastasis. After the diagnosis, thorax CT and bronchoscopy were performed, but revealed no pathology.

- Brain CT revealed the edematous regions at the inferior section of both parietal lobes
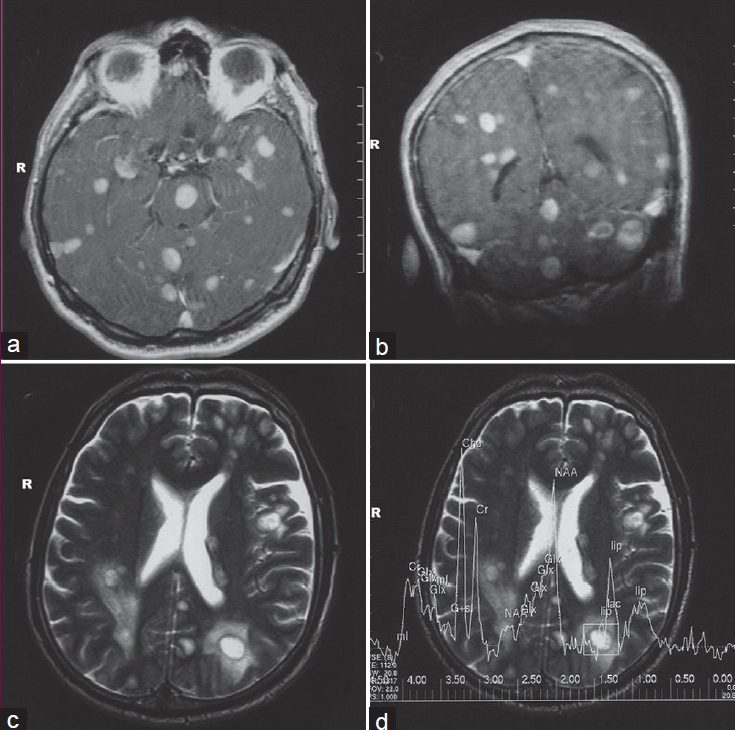
- Gadolinium-enhanced axial, and coronal T1-weighted MRI section revealed multiple millimetric nodular lesions with homogenous enhancement in both cerebral hemispheres and the brainstem (a, b), Axial T2-weighted MRI of the brain revealed multiple nodular lesions associated with surrounding edema (c), Proton MRS showed an increase in choline peak (d)
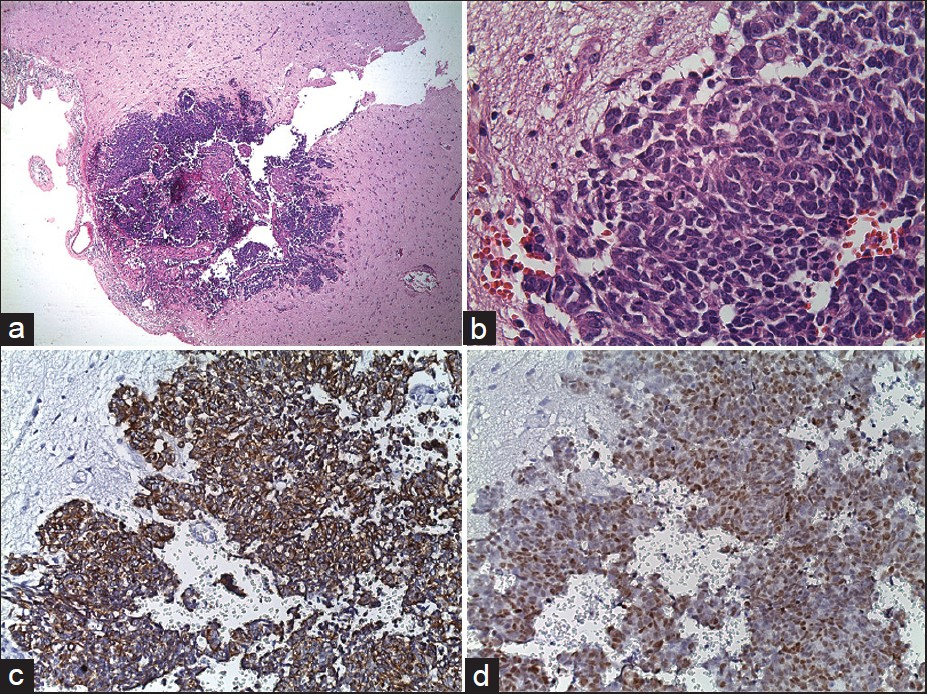
- Pathologic specimen of cerebral biopsy. Microscopic examination with H and E staining revealed infiltrating glial tissue by neoplastic cells (original magnification ×4) (a), Microscopic examination with H and E staining revealed solid nests of atypical cells having pleomorphic nuclei and eosinophilic cytoplasm (original magnification ×40) (b), Immunohistochemical examination of tissue sections showed neoplastic cells cytoplasms staining positively for cytokeratin 7, and nuclear TTF-1 expression of neoplastic cells (c, d)
The patient's general medical condition was rapidly deteriorated under medical treatment and he died within a one month following whole brain irradiation (2000 cGy/5 fractions/1 week). Requested autopsy was declined by his family.
Discussion
Miliary form of tumor metastasis throughout the brain has been firstly described by Madow and Alpers in 1951 with the term of “carcinomatous encephalitis” based on the clinical and histopathological findings.[5] This condition is usually seen as an advanced stage of the primary disease, and the survival time of the patients with miliary brain metastases are fairly limited.[1] Investigations regarding the primary focus of miliary brain metastases have revealed that the majority of cases originated from a lung carcinoma because of easy accessibility of the systemic arterial circulation by tumor cells in the lung.[16] In the treatment of such lesions, whole-brain radiotherapy (WBRT) is still the standard option. Although, gefitinib has been reported to be a useful additive drug for resolution of the multiple metastatic lesions; even though further researches are still necessary.[6–8]
Clinical manifestations of patients with miliary brain metastases are usually silent and differ from patient to patient. A variety of neurological deficits ranging from short and long term memory defects to hemiparesis may be present. However, neurological findings can strangely be minimal in a majority of patients with multiple lesions, perhaps due to weak edematous effects of these masses.[9] There was no neurological deficit in our case except for limited cooperation.
Plain skull films and brain CT have limited diagnostic value as calcification is rarely seen in these tumors. They are seen as nodular millimetric lesions with perivascular spreading, showing iso- to low-intensity signals on T1- and high-intensity signals on T2-weighted sequences of MRI, and present a nodular or peripheral (ring-like) contrast enhancement following gadolinium injection.[1] However, a large number of infectious and non-infectious diseases such as miliary brain tuberculosis, neurocysticercosis or toxoplasmosis, can cause multiple enhancing lesions in the brain with the dominance of infective pathologies.[3] All of these circumstances contribute to scarcity of useful diagnostic information about this condition.
Recently discovered proton MRS imaging technique is a potent instrument to analyze and monitor tissue metabolism noninvasively. It reflects alterations of the intracellular metabolite concentrations, such as Cho, Cr, NAA, lactate and lipids on pathologic tissue that differs from normal brain tissues.[4] On proton MRS, non-neoplastic lesions such as cerebral infarctions and brain abscesses had noticeable decreases in Cho, Cr, and NAA levels while tumors have generally elevated Cho and decreased levels of Cr and NAA. Mφller-Hartman et al,[10] reported that intracranial metastatic lesions showed strongly elevated lipid levels on proton MRS, with statistically significant differences from all other intracranial mass lesions, except tuberculosis abscess origin, or in cases of toxoplasmosis. However, brain abscesses showed decreased levels of Cho, Cr and NAA. Recognition and prompt diagnosis of other contrast enhanced miliary brain lesions are important because early treatment can prevent patient from worsening, and lead to clinical improvement. Proton MRS may also serve as a diagnostic tool to differentiate miliary brain metastases arise from different unknown origins. Thereby, it may reduce an extended search for diagnosing of a primary unknown neoplasm and number of diagnostic biopsies has been used for differential diagnosis.[11]
A histopathologic examination is important for diagnosis; especially in patients with primary tumor focus cannot be found with other studies. To our knowledge, only 17 patients with pathologically proven diagnosis of miliary brain metastases have been reported until now.[16] The most common histological type was adenocarcinoma where, lung was the most common primary site of origin.
Thyroid transcription factor-1 (TTF-1) is a tissue-specific transcription factor which is expressed by epithelial cells in the thyroid and the lung. TTF-1 is a very sensitive and specific marker to document the pulmonary origin of an adenocarcinoma. The combinations of CK7 positive/CK 20 negative immunophenotype along with TTF-1 immunoreactivity were highly specific for primary pulmonary adenocarcinoma. In our case, immunohistochemical examination showed that neoplastic cells were positive for cytokeratin 7 and TTF-1 whereas cytokeratin 20 and surfactant were negative. Depending on the immunohistochemical findings, lung was considered to be a possible primary site of origin and moderately or poorly differentiated adenocarcinoma of the lung was considered as histological subtype.
The clinical, radiological and pathological features of miliary metastatic tumors have not been clarified yet, and there are no definite data on the stages and spread phases of the lesions in the brain. Despite the advances in technical equipment and medical knowledge the differential diagnosis of miliary metastatic brain tumors is difficult. Distinguishing non-neoplastic lesions from neoplastic lesions are extremely important because a misdiagnosis can lead to unwarranted neurosurgery and exposure to toxic chemotherapy or potentially harmful brain irradiation. Nowadays, the pathological examinations of the tissue samples are still the gold standard for the diagnosis of miliary brain metastasis. However, proton MRS may contribute to make a differential diagnosis.
Source of Support: Nil
Conflict of Interest: None declared
References
- Miliary brain metastases from adenocarcinoma of the lung: MR imaging findings with clinical and post-mortem histopathologic correlation. Neuroradiology. 2007;49:35-9.
- [Google Scholar]
- Carcinomatous encephalitis as clinical presentation of occult lung adenocarcinoma: Case report. Arq Neuropsiquiatr. 2007;65:841-4.
- [Google Scholar]
- A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. AJNR Am J Neuroradiol. 2006;27:1404-11.
- [Google Scholar]
- Encephalitic form of metastatic carcinoma. AMA Arch Neurol Psychiatry. 1951;65:161-73.
- [Google Scholar]
- Miliary brain metastasis presenting with dementia: Progression pattern of cancer metastases in the cerebral cortex. Neuropathology. 2007;27:390-5.
- [Google Scholar]
- Epidermal growth factor receptor targeted therapy by ZD 1839 (Iressa) in patients with brain metastases from non-small cell lung cancer (NSCLC) Lung Cancer. 2003;41:227-31.
- [Google Scholar]
- Gefinitib in patients with brain metastases from non-small cell lung cancer: A prospective trial. Ann Oncol. 2004;15:1042-7.
- [Google Scholar]
- Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44:371-81.
- [Google Scholar]
- Metobolic profiling of human brain metastases using in vivo proton MR spectroscopy at 3T. BMC Cancer. 2007;7:141.
- [Google Scholar]






